It is a hospital based study focusing on epidemiological aspects of vitiligo and association with autoimmune disorders. There are few studies elucidating the association of autoimmune disorders with vitiligo in the Indian population. Our study is a small attempt in this direction.
AimTo study epidemiological parameters of vitiligo and to study coexistence of autoimmune disorders.
Materials and methodsRecords of 33,252 new patients attending the dermatology outpatient department from June 2002 to June 2008 were analysed for the presence of vitiligo and details of important epidemiological variables, and associated autoimmune disorders of these patients were collected and analysed.
ResultsTotal number of vitiligo patients was 204. Proportion of vitiligo patients was 0.61%. Male:female proportion was almost equal. Family history of vitiligo was seen in 3.43% of cases.
Associated autoimmune disorders were seen in 2.94% cases and were mainly skin associated autoimmune diseases (morphoea, alopecia areata, discoid lupus erythematosus, and pemphigus erythematosus) except for one case of Grave's disease.
ConclusionAssociation of vitiligo with other autoimmune diseases emphasizes autoimmune aetiology of vitiligo. This study also emphasizes the need to actively look for, and if necessary, investigate patients with vitiligo for other autoimmune diseases.
Vitiligo is characterised by the presence of depigmented macules due to the destruction of cutaneous melanocytes. The psychological impact of vitiligo on the patient can be tremendous. The exact aetiology remains obscure, but several hypotheses have been implicated: neural, autoimmune and cytotoxic.1–3 The autoimmune aetiology of vitiligo is the most widely accepted, especially for generalised vitiligo, and forms the basis of several therapies. Frequency and type of autoimmune diseases associated with vitiligo is variable as is evident in different studies/surveys conducted across the world, probably because of the different patient populations studied.4–8 Also, studies have shown increased frequency of same autoimmune diseases in first degree relatives of the patients studied.4 Such data indicates that individuals can be genetically predisposed to a specific group of autoimmune disorders that includes vitiligo. Our study is an attempt to focus on the coexistence of autoimmune disorders in vitiligo.
AimTo study epidemiological parameters and coexistence of autoimmune disorders with vitiligo.
Objectives- (1)
To study prevalence of vitiligo in patients attending the dermatology outpatient department (OPD) during the years June 2002 to June 2008.
- (2)
To study demographic variables associated with patients having vitiligo.
- (3)
To study the coexistence of autoimmune disorders with vitiligo.
Records of 33,252 new patients attending dermatology OPD between June 2002 and June 2008 were analysed retrospectively for the presence of vitiligo and coexistence of autoimmune disorders as per history and examination. The decision to select time period was random.
Structured proforma was devised, and standardised information about individual subject including demographic variables and associated autoimmune diseases was collected, once the record showed the presence of vitiligo. Since our department has a vitiligo clinic, detailed information about all vitiligo patients is maintained in a register and was easily retrieved.
A master chart was prepared for detailed information about vitiligo patients. To estimate overall prevalence of vitiligo in new patients attending dermatology OPD 95% confidence interval of prevalence of vitiligo was calculated. Patients were categorised as per their demographic variables, i.e. age, sex, type of vitiligo, presence of associated autoimmune disorders, family history of vitiligo (in first degree relatives) and family history of associated autoimmune disorders. Depending on the nature of associated autoimmune disorder, information about necessary investigations (e.g. skin biopsy, hormonal tests) was also scrutinised to confirm the diagnosis of the associated autoimmune disorder.
Results- 1.
Demographic characteristics: The total number of vitiligo patients studied was 204. The proportion of vitiligo patients was 0.61% (95% confidence interval was 0.53–0.69%). Age distribution: Age group of 10–20years accounted for maximum number of patients (30.39%). Mean age was 24years. The youngest patient observed having vitiligo was six months old and the oldest patient observed was 79years old. Females (104) constituted 51% and males (100) were 49% (M:F ratio 1.04:1).
- 2.
Family characteristics: Family history of vitiligo in first degree relatives was found in 3.43% of patients. There was no family history of any autoimmune disorders.
- 3.
Clinical features of vitiligo: 66% cases were found to have localised vitiligo (<20% of body surface involvement), 15% had generalised vitiligo. 13% had acral/acromucosal/mucosal vitiligo and the least common type observed was segmental vitiligo (6%).
- 4.
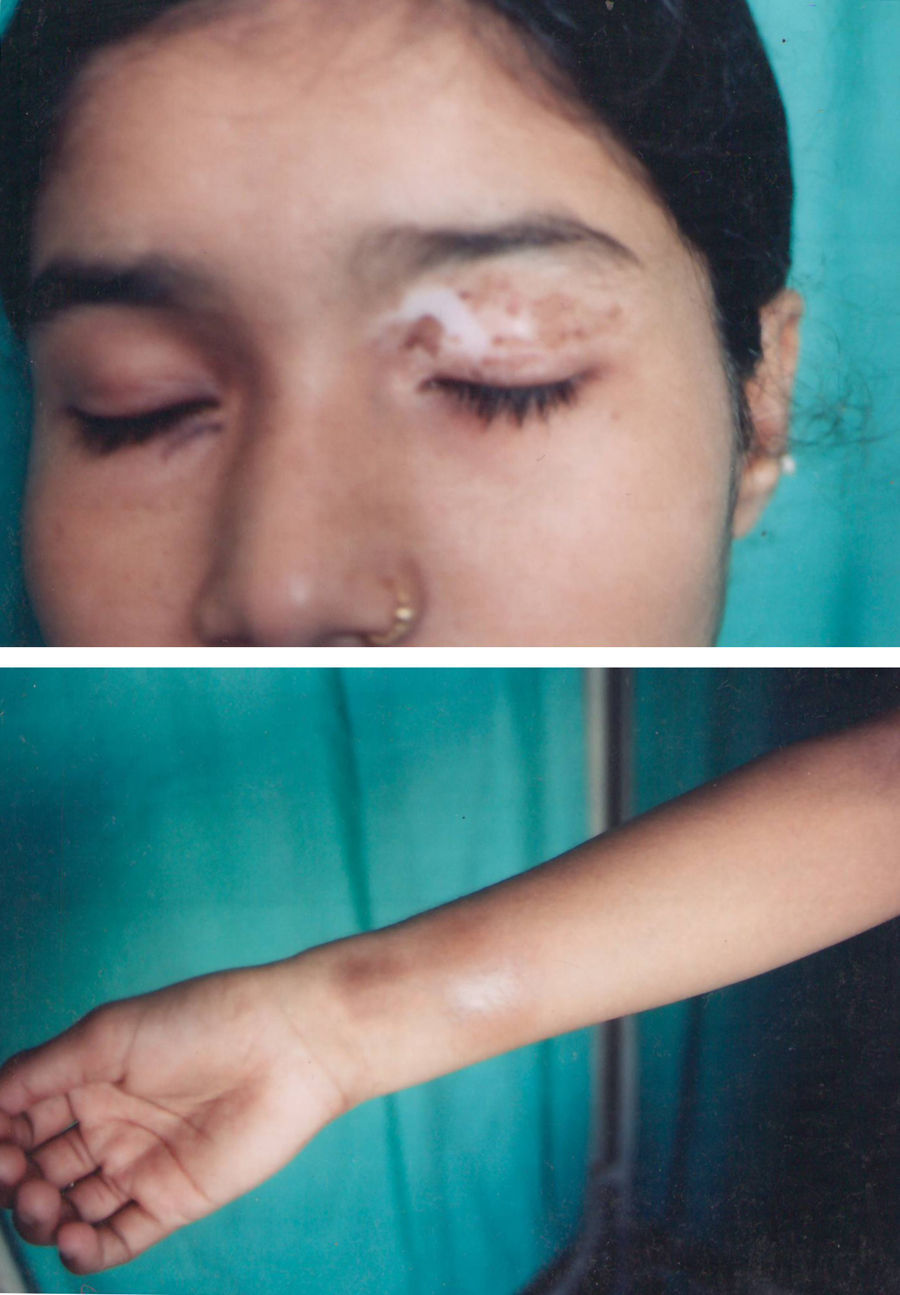
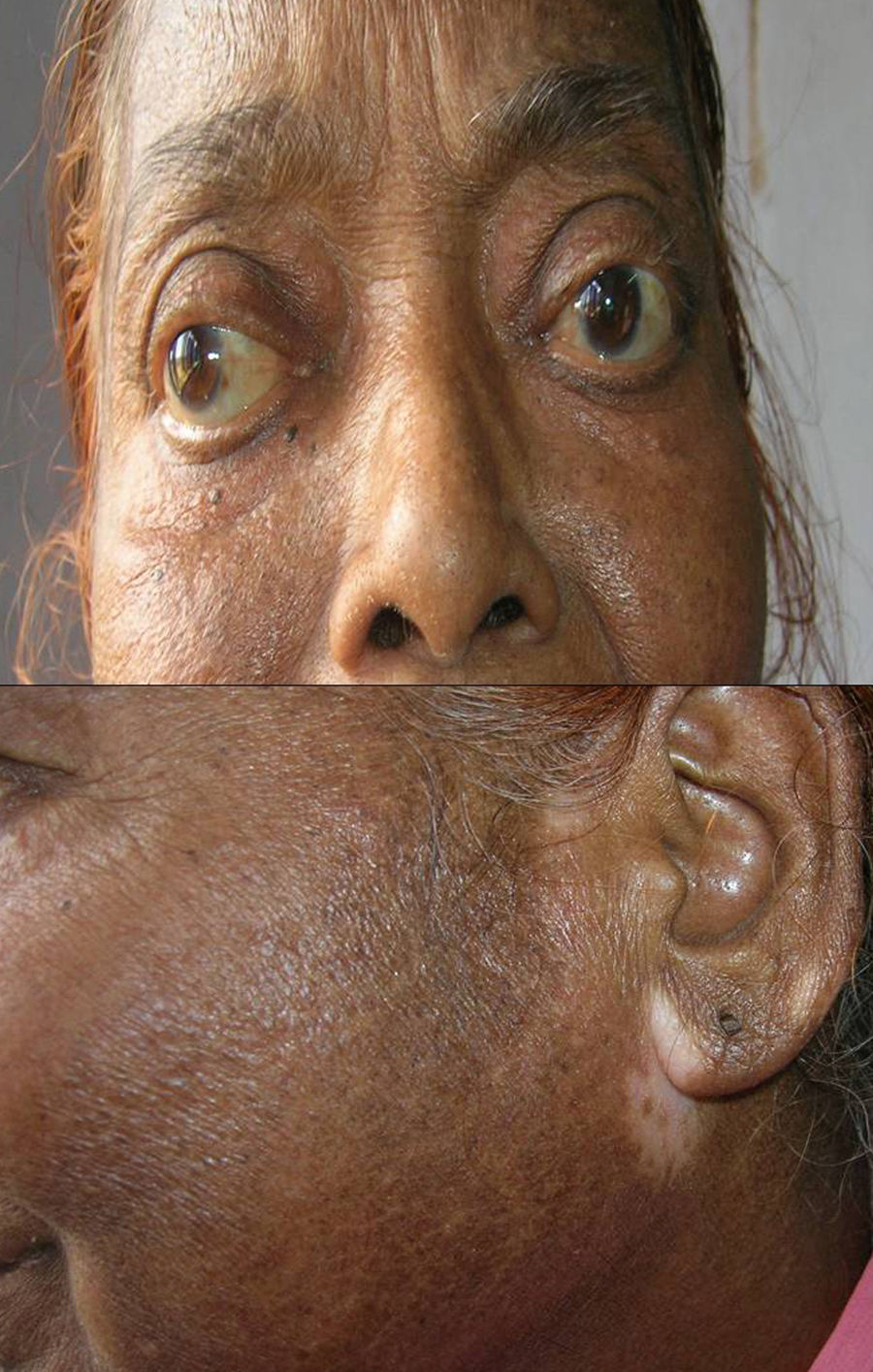
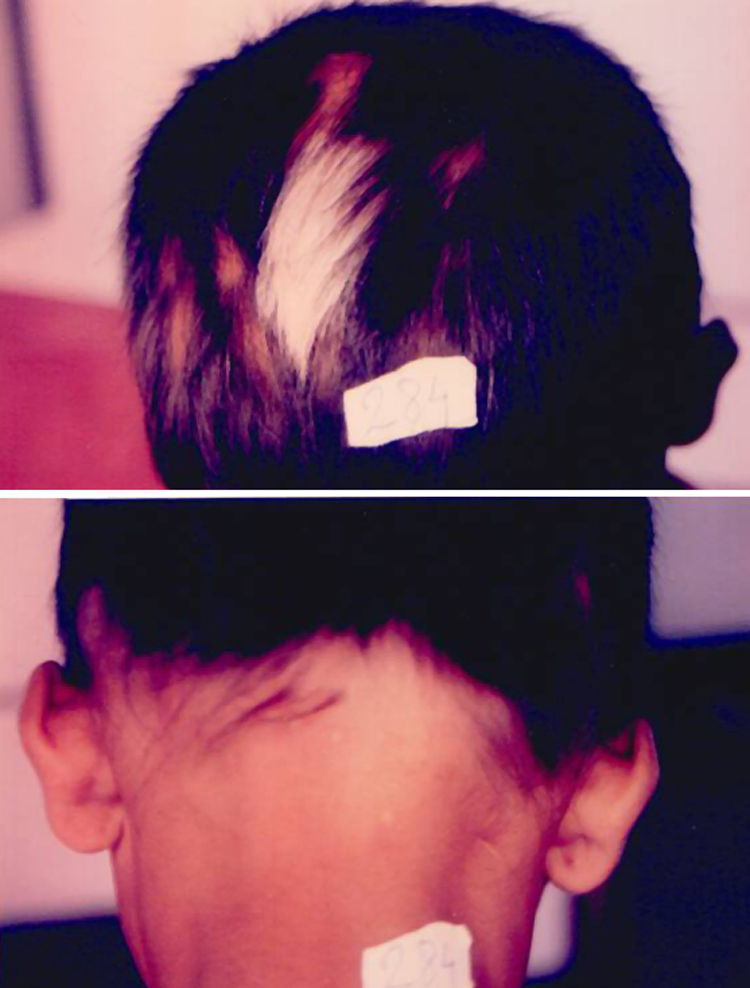
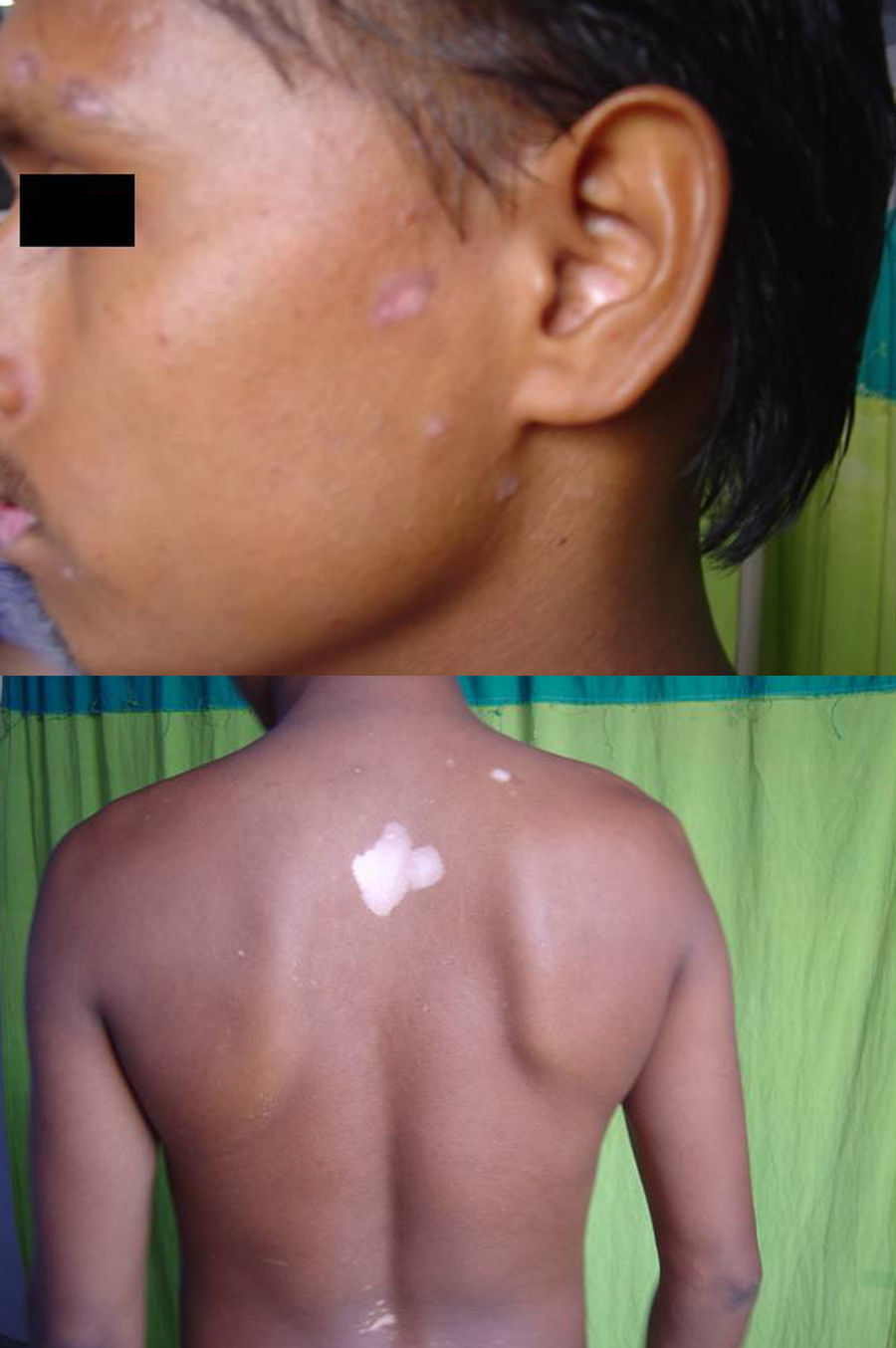
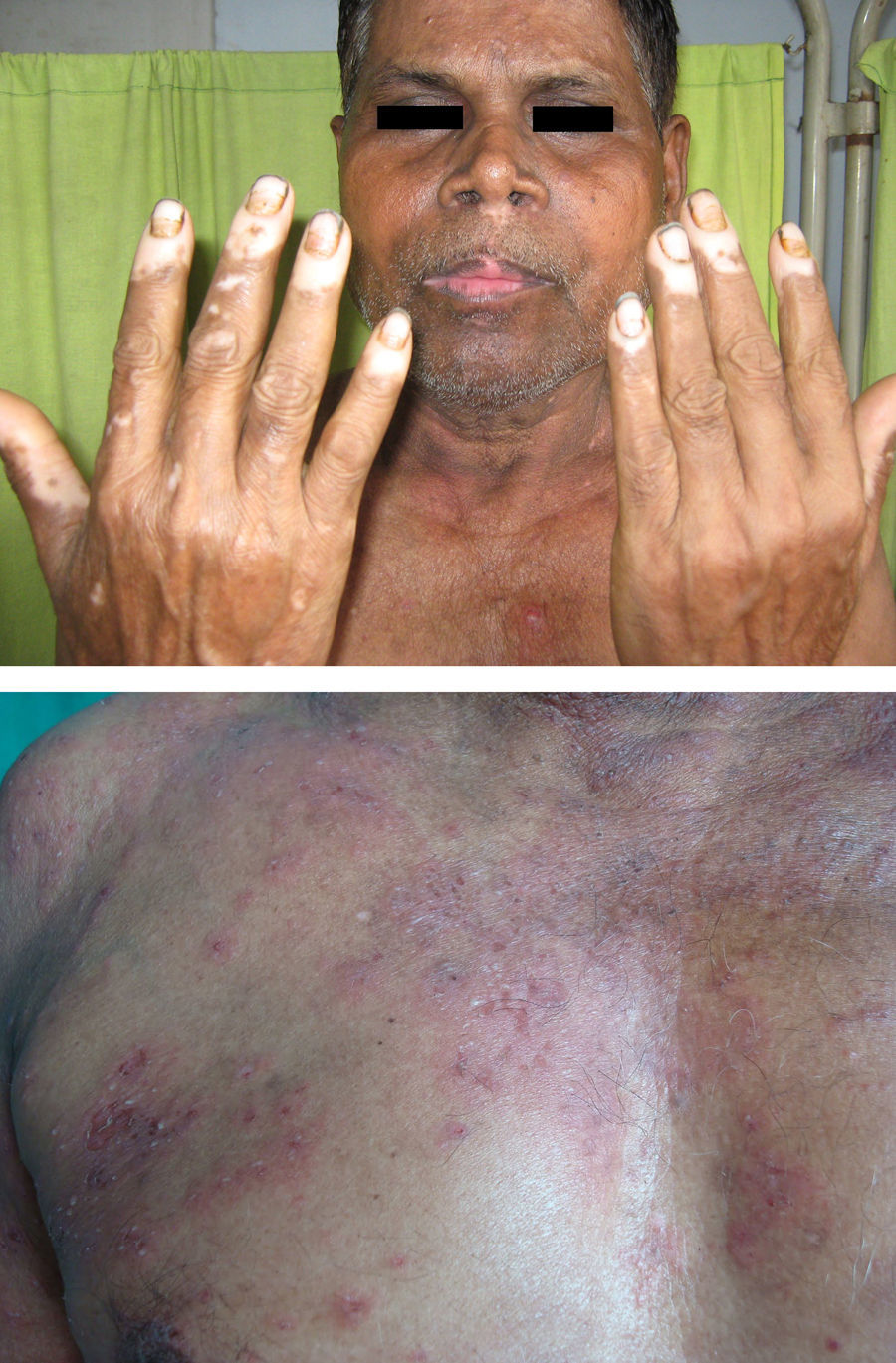
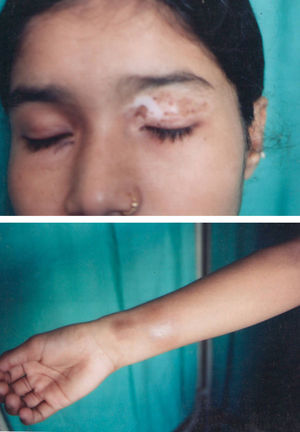
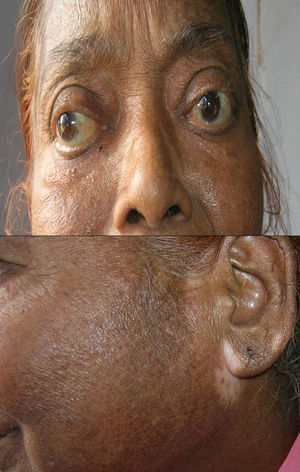
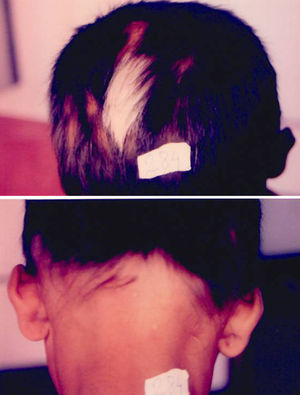
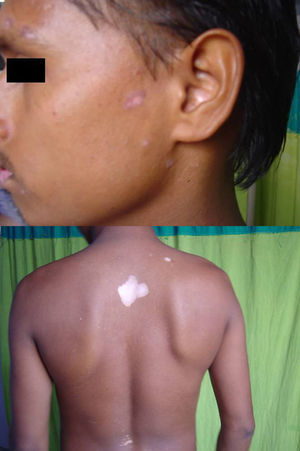
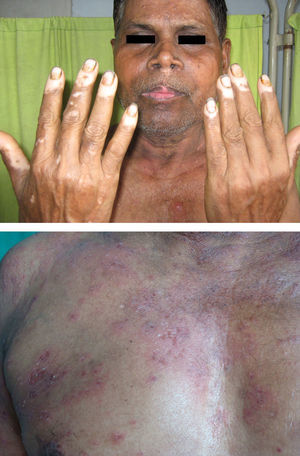
Associated autoimmune disorders were present in 2.94% of the patients (Table 1). These were two cases of morphoea (0.98%) (Fig. 1), and one case each of Graves’ disease (0.49%) (Fig. 2), alopecia areata (0.49%) (Fig. 3), discoid lupus erythematosus (0.49%) (Fig. 4), and pemphigus erythematosus (0.49%) (Fig. 5). Of these, two patients belonged to an older age group (>40years) and four patients belonged to younger age group (<20years). Three patients had localised vitiligo, one patient had generalised vitiligo, one patient had acromucosal vitiligo and one patient had mucosal vitiligo.
Table 1.Associated autoimmune disorders.
Serial no. Type of associated autoimmune disorder Age/sex of the patient Type of vitiligo Percentage 1 Morphoea 18/F Localised 0.98 2 Morphoea 3/M Mucosal 3 Alopecia areata 14/M Localised 0.49 4 Discoid lupus erythematosus 19/M Localised 0.49 5 Pemphigus erythematosus 65/M Acromucosal vitiligo 0.49 6 Grave's disease 58/F Generalised 0.49
Vitiligo is a multifactorial disorder, autoimmune hypothesis being the most important in the pathogenesis of vitiligo. The autoimmune hypothesis of vitiligo primarily focuses on: (1) association with other autoimmune disorders; (2) association with family history of vitiligo and autoimmune disorders; (3) presence of autoantibodies to melanocytes and autoreactive T cells; (4) presence of genetic factors, i.e. MHC class II alleles, cytotoxic T lymphocyte antigen 4 gene, autoimmune susceptibility foci; and (5) positive response to immunosuppressive therapeutic agents.
Vitiligo is associated with the following autoimmune diseases: (1) autoimmune polyendocrinopathy syndrome type 1 (APS1), type 2 (APS2, Schmidt syndrome), type 3 and type 4. Vitiligo is more frequently a component of APS 3.9 (2) Autoimmune thyroiditis, Graves’ disease,10,11 (3) Addison's disease,12 (4) Pernicious anaemia,4 (5) Myasthenia gravis,4 (6) Alopecia areata,4 (7) Pemphigus vulgaris and foliaceus,13,14 (8) Morphoea,15 (9) diabetes mellitus type 1,4 (10) rheumatoid arthritis,4 and (11) systemic lupus erythematosus and discoid lupus erythematosus.4,16
Alkhateeb et al. reported at least 30% of patients with vitiligo to be affected with at least one additional autoimmune disorder.4 His study on Caucasian probands and their families reported a significant increase in thyroid disorders, Addison's disease and systemic lupus erythematosus in vitiligo probands as well as their families as compared to the population frequency, while there was no significant increase in alopecia areata, diabetes mellitus, myasthenia gravis and rheumatoid arthritis. The commonest association of vitiligo is with thyroid dysfunction, especially autoimmune thyroiditis. The incidence of thyroid dysfunction reported is varied in different studies: Betterle et al. reported 7.5%; Zettining et al. 21%; Shah et al. 0.27%; Tanioka et al. (7.4% of generalised vitiligo patients).7,17,18 Dave et al. reported abnormal thyroid profile in as much as 40% of vitiligo patients and also higher incidence of thyroid dysfunction in mucosal vitiligo.6 Some studies have reported subclinical thyroid disease and some have reported only the presence of anti-thyroid peroxidase antibodies, thyroid microsomal antibodies and antithyroglobulin antibodies.17,18 Increased frequency of autoimmune diseases has also been specifically described in family members of multiplex vitiligo families (families with multiple members having vitiligo), thus reflecting an inherited genetic component of autoimmune susceptibility in these families.19
Apart from statistically significant association, several immunogenetic factors provide a scientific basis of a true association of vitiligo with other autoimmune disorders: (1) autoimmune susceptibility foci20,21: genome analysis has mapped vitiligo to specific loci, termed as autoimmune susceptibility loci: AIS1 (chromosome 1p31.3–32.2), AIS2 (chromosome 7), AIS3 (chromosome 8), SLEV1 (17p13 – a linkage signal detected in multiplex lupus families that included at least one case of vitiligo). The linkage on chromosome 8 appears to derive from families with isolated vitiligo, while 7 and 17p linkages appear to derive from families with other autoimmune disorders and may represent loci for susceptibility in general. Association analysis across the 17p linkage region has also identified NALP1 as a major susceptibility gene for generalised vitiligo and other autoimmune and autoinflammatory diseases associated with vitiligo.22 NALP1 recruits the adaptor protein ASC, caspase 1 and caspase 5 to a complex termed the NALP1 inflassome, which activates ILβ. Serum ILβ levels are elevated in patients with generalised vitiligo. NALP1 also plays a role in caspase mediated cellular apoptosis. If a definitive role for NALP1 and ILβ is confirmed, ILβ inhibitors (anakrina) and caspase 1 inhibitors (VX-765) might be effective in the treatment or in the prevention of autoimmune disorders like vitiligo.23,24 (2) An increased frequency of heterozygous C4 and C2 deficiency has been reported in patients with vitiligo. C4 and C2 deficiency is known to be associated with several autoimmune disorders.25 (3) Cytotoxic T lymphocyte antigen 4: Significant association with CTLA 4 polymorphic markers is only seen in patients of vitiligo who have concomitant autoimmune disease.26 (4) Autoimmune regulator: mutations in the autoimmune regulator gene (AIRE) are responsible for autoimmune polyendocrine syndrome type 1(APS1), a disease in which vitiligo is a common clinical manifestation.5 (5) Lymphoid protein tyrosinase phosphatase: mutations in the gene (PTPN22) encoding lymphoid protein tyrosine phosphatase have an influence on the development of generalised vitiligo and have been associated with susceptibility to autoimmune disorders.27 All of these provide further evidence for autoimmunity as an aetiological factor in vitiligo.
Our study shows a proportion of 0.61% of vitiligo patients amongst new patients. This is similar to the prevalence of 0.5% reported by Das et al. in Kolkata, India.28 Shah et al. reported an incidence of 1.84% amongst new patients.8 Mean age of the patients was 24years and there was an equal proportion of males and females. Family history was seen in 3.43% of patients, which is less than that reported by Shajil et al. (13.68%) and Gopal et al. (22%).29,30
Associated autoimmune diseases in our study constituted 2.94% and were mainly cutaneous associated autoimmune diseases and only one case of Grave's disease. Amongst the cutaneous autoimmune diseases, morphoea constituted 0.98%; alopecia areata 0.49%; pemphigus erythematosus 0.49%; and discoid lupus erythematosus 0.49%. Shah et al. have reported autoimmune diseases in 1.36% of vitiligo patients. Other studies have reported increased frequency of thyroid diseases especially Hashimoto's thyroiditis.4,7,17 Grave's disease has been reported in association with vitiligo by Moradi and Ghafarpoor, Dave et al. and Tanioka et al.6,7,10 Gopal et al. reported alopecia areata in 7.4% and Shah et al. in 0.55% of vitiligo patients.8,30 To the best of our knowledge, our case is the first case of pemphigus erythematosus to be reported in association with vitiligo, although pemphigus foliaceus/vulgaris and lupus erythematosus have been reported with vitiligo.13,14,16
The associated autoimmune diseases were more in the younger age group (<20years) – four patients than in older age group – two patients (> 40years). Also, three of these patients had localised vitiligo, one patient had acromucosal vitiligo, one patient had mucosal vitiligo and only one patient showed generalised vitiligo, while other studies have shown increased frequency of autoimmune diseases in generalised vitiligo.7
Our study has a relatively small sample size, hence we cannot comment on the statistical significance of the associated autoimmune diseases. Also it is a retrospective study. If it had been a prospective study, we could have also included a few basic investigations for other autoimmune disorders like thyroid disorders even in asymptomatic patients. This probably explains the low proportion of thyroid disorders in our study. Presently, we have started screening all patients of vitiligo in our hospital for thyroid disorders (T3, T4, and TSH). Nevertheless, our study once again emphasizes the autoimmune aetiology of vitiligo and a possible genetic basis of concurrent autoimmune diseases in such patients.
We suggest that:A genomic analysis should be done in patients of vitiligo with other autoimmune diseases, but in a larger sample size in order to identify genes responsible for multiple autoimmune diseases including vitiligo in the Indian population. This has the potential to yield novel insight into the pathogenesis of these diseases and may lead to identification of new targets for therapy and possibly prevention.A detailed clinical examination should be carried out along with a few basic investigations and auto-antibodies especially, antithyroid antibodies in patients with vitiligo for early detection of coexisting auto immune disorders, especially thyroid diseases.
Conflicting interestThe authors have no conflict of interest to declare.