INTRODUCTION
Recurrent acute respiratory tract infections (RARTIs) in children are related to IgG subclass deficiency, especially to IgG2 deficiency. These deficiencies may be related to the incapacity to mount effective immune responses to polysaccharides1-4. The humoral immune response to pneumococcal polysacccharides is restricted to IgG2, and protective immunity to bacteria-bearing polysaccharides is mediated by IgG2 antibodies1-5.
The prevalence of these deficiencies in children suffering RARTIs varies from 25 % to 63 %; IgG1 from 1 % to 25 %; IgG2 from 11 % to 44 %; IgG3 from 0 % to 21 %; and IgG4 from 0 % to 38 %1. These children are not able to mount normal immune responses to immunization with plain polysaccharides4,6, but they present immune responses similar to those of normal children if they are immunized with polysaccharides conjugated to proteins7-9. Similar conditions, IgG subclass deficiencies related to RARTIs and chronic respiratory infections, have also been described in adults10-13.
It is important to note that immune alterations and the associated RARTIs are reversible, i.e., the patients may present clinical and immune improvement over time14,15.
OM-85 BV (Broncho-Vaxom, OM PHARMA, Geneva Switzerland, marketed in Mexico by Química Knoll de México) is an immunostimulant for the prevention of ARTIs. OM-85 BV is the product of alkaline proteolysis from lysates of the following bacteria; Haemophilus influenzae; Streptococcus pneumoniae; Klebsiella pneumoniae; Klebsiella ozaenae; Staphylococcus aureus; Streptococcus pyogenes; Streptococcus viridans; Morexella catarrhal is16. Each strain contributes the same relative proportion to the total protein.
OM-85 BV is free of toxic lipopolysaccharides (LPS); therefore, it does not act on LPS receptor CD 1417. In macrophage and monocytic cells, OM-85 BV increases intracellular calcium levels and induces the production of glucose-regulated protein (GRP78)18 and C-Fos/SRE protein19,20. These second messengers induce the expression of pro-inflammatory cytokines IL1-α, IL-6, IL-8, and TNF-α19-22. In vitro experiments indicate that OM-85 BV exerts its action through the signal transducer gp 130 and the gp 130 binding cytokines IL6, IL11, and IL1223. Additionally, OM-85 BV induces phagocytic cells to produce NO and O222 and to express adhesion molecules17,24. Patients receiving OM-85 BV have shown enhancement of cellular immune responses25,26; increase in secretory IgA27-29, serum IgA30,31, serum IgG, and serum IgM27,31 and activation of phagocytic cells28,29.
OM-85 BV has shown safety and efficacy in the prevention of ARTIs in exposed children attending day-care centers32 and orphanages33 and in highly susceptible children34,35. OM-85 BV has been shown to have some effect on children suffering from immune system defects such as IgG or IgA deficiency and common variable immunodeficiency36,37. The aim of the trial is to prove the effect of OM-85 BV on the IgG subclass levels in children suffering RARTIs and exhibiting IgG subclass subnormal levels as well as on the number of RARTIs.
PATIENTS AND METHODS
A placebo-controlled, double blind, parallel, and prospective trial was conducted. The patients were children from three to six years of age living in the metropolitan area of Mexico City. The trial participants were outpatients attending to the external consultation of the Hospital Infantil de México.
Sample size calculations were performed with Primer on Statistics 3.0 software (Mc-Graw-Hill, New York NY). According to clinical parameters, we estimated a sample size of 23 patients per group, considering the previous trials in Mexico, with an incidence of 2.99 ± 0.81 (mean ± SD) ARTIs in the placebo group during six months and a 50 % reduction in the OM-85 BV group33.
The selection criteria were as follows; at least 3 ARTIs (based on the number of medication prescriptions) in the previous six months, with no anatomic alterations of the respiratory tract by physical examination, chronic respiratory diseases (tuberculosis, cystic fibrosis), autoimmune diseases, liver failure, kidney failure, malnutrition, or cancer and no treatment with corticosteroids, immunosuppressants, immunostimulants, gammaglobulins, or anticonvulsive drugs in the last six months.
Informed consent for each participant was obtained from the parents at entry. Children also gave their oral consent. The protocol and the case report form were approved by the local committee of investigation and ethics and were performed according to the Mexican regulation and the Helsinki Declaration of 1975, as revised in 1983.
After completion of clinical selection criteria and acknowledgment of informed consent, serum IgG subclasses were assessed. Levels of serum IgG subclasses were determined by enzyme immunoassay (Bindazyme TM, Human IgG Subclass combi kit, The Binding Site LTD, Birmingham, UK) according to the manufacturer's directions. A standard control curve was run for each set of determinations. Determinations were performed before the beginning of the trial medications and ten days after the last trial drug administration. The timing of the second sampling was based on a previous clinical trial determining total serum IgG27.
Values less than 422 mg/dl for IgG1, 117 mg/dl for IgG2, 41 mg/dl for IgG3, and 15 mg/dl for IgG4 were considered subnormal. These levels are below the 95 % confidence limits reported by Schurr38 for IgG4 and below the 95 % confidence limits reported by Oxelius39 for the rest of IgG subclasses. Similar values were used recently by Popa10.
Only the children with subnormal levels of at least one IgG subclass were randomized as consecutive numbers were assigned to patients. The numbers had been previously randomized to the treatment groups in balanced blocks of 10. The treatment for each patient number was prepared in advance. The boxes, blisters, and capsules had the same appearance and the taste of the powders was similar. Investigators, laboratory technicians, parents and patients were all blinded to the identity of the capsules.
The patients orally ingested one capsule (or the powder contained in the capsule) of OM-85 BV (3.5 mg) or placebo per day for ten consecutive days per month during three consecutive months. The parents administered the capsules, and the empty blisters were kept to control compliance. The patients were followed for another three months after this treatment to complete a total trial period of six months.
The medication codes were enclosed in opaque sealed envelopes and kept available for the researcher in the study center to be opened in case of a serious adverse event.
Patients were assessed monthly and every time they presented respiratory symptoms, and all the ARTIs were followed up to the resolution of the clinical picture. All the physical examinations and drug prescriptions were made by Dr. Del-Rio-Navarro and Dr. Avila-Castañón. Antibiotics were prescribed when purulent secretions were present or in the case of otitis or lower ARTI.
An upper ARTI was defined as the presence of at least one of the following signs; rhinorrhea, sore throat, or cough for 48 hours or more without signs of lower ARTI. Lower ARTI was defined as the presence of at least one of the following signs; rales or crepitations, wheezing, stridor, respiratory rate > 50/min, cyanosis, or chest retractions for more than 48 hr. Otitis was defined as acute onset of earache with erythema and limited mobility of the tympanic membrane determined by pneumatic otoscopy. Similar upper and lower ARTI definitions have been used in epidemiological studies in developing countries40. Additionally, rhinitis was defined by the presence of nonpurulent rhinorrhea without other symptoms and sinusitis as purulent nasal or retronasal discharge with nose congestion, cough, halitosis, and facial pain41. Two infections were counted as such only when the patient was without symptoms for at least 72 hours between the end of one episode and the beginning of the next.
The trial began in December 1998 and was completed by March 2001. Patients were recruited from December 1998 to October 2000. Adverse events were registered in clinical files and in the adverse report form as they occurred and were reported monthly in the case report form. The trial medications and case report forms were provided by Química Knoll de México SA de CV BASF Pharma.
The number of ARTIs at the end-point was evaluated using Student's t test and Mann-Whitney U test. The statistics for IgG subclasses was non-parametric as the values had a non normal distribution. The changes in the IgG subclass levels were assessed in each treatment group using Wilcoxon test; this test takes in account the directions of individual changes and magnitude of such changes. SPSS statistics software was employed for these calculations. Median differences, the recommended way to represent these differences in non-parametric statistics, as well as the corresponding upper and lower quartiles were calculated with the Confidence Interval Analysis software, version 2.0. Additionally the pattern of kind of illness was assessed by chi square test, and the relation between IgG subclasses levels and number of infections was explored by simple correlation, as well as the IgG subclasses levels according to the number of infections and number of infections according to the number of infection and IgG subclasses levels by Mann-Whitney U test.
RESULTS
Three hundred children were screened; 63 patients had the clinical selection criteria and 54 were randomized, but it was found that five of them had, in fact, normal levels of IgG subclasses (due to clerical mistake these patients were randomized before the IgG subclasses report was delivered), which left only 49 evaluable patients.
There were 25 children in OM, but three children were lost to follow-up leaving 22 children with two determinations of IgG subclasses. Then two other children were lost to follow-up before they completed the total study period of six months.
There were 24 children in the placebo group, but one patient was lost to follow-up, another retired the informed consent, and another was withdrawn due to an adverse event leaving 21 children with two determinations of IgG subclasses. Then another child was lost to follow-up before he completed the total study period of six months.
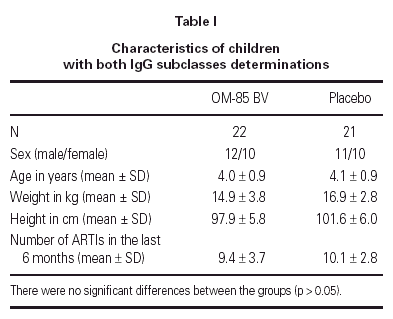
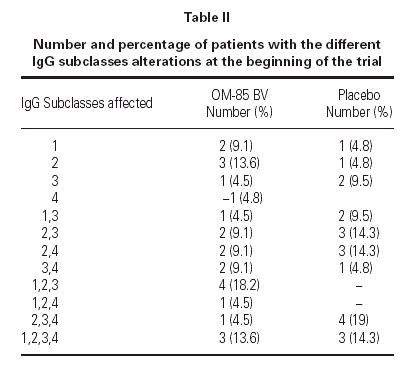
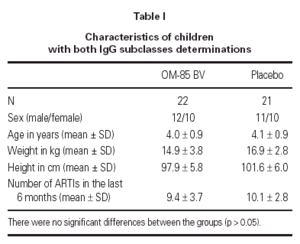
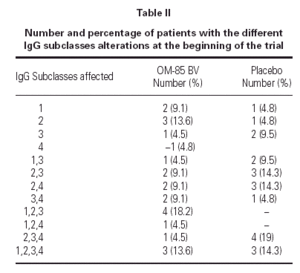
The demographics of the children with both IgG subclass determinations are in table I. The pattern of IgG subclass alterations is presented in table II. Most of the patients presented alteration in two or more IgG subclass levels.
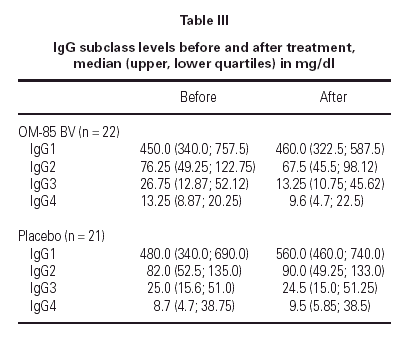
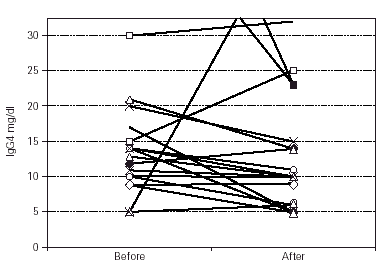
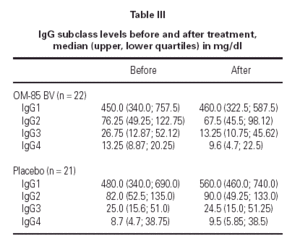
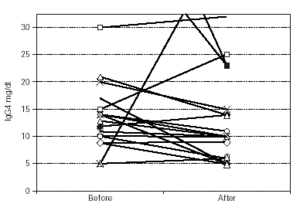
Median IgG subclass levels before and after trial treatment are represented in table III. The levels of IgG4 diminished in the OM-85 BV group (-3 [-8.0, -1.0] median difference [95 % CI] p < 0.05 by Wilcoxon test). Reduction in IgG4 levels was recorded in 14 out of 22 patients. No other significant changes were observed, but there was a trend to IgG3 reduction in the OM-85 BV group. Figure 1 represents the individual changes in the IgG4 levels. There were no differences between OM-85 BV and placebo groups before and after the treatment.
Figure 1.--Individual levels of IgG4 before and after treatment.
The OM-85BV group statistics revealed 56 ARTIs; 25 rhinitis; 23 Upper ARTIs; 2 otitis; 1 sinusitis; 3 Lower ARTIs; 1 sinusitis plus Lower ARTI; and 1 otitis plus Lower ARTI. The placebo group suffered 104 ARTIs; 30 rhinitis; 47 Upper ARTIs; 2 otitis; 15 sinusitis; 9 Lower ARTIs and 1 otitis plus Lower ARTI. The pattern of illness is different in both groups (p < 0.05 by chi square); the main difference is the relative proportion of rhinitis (45 % of total infections in OM85-BV vs 29 % of total infections in the placebo group) and sinusitis (2 % vs 14 % respectively).
The OM-85 patients experienced 2.8 ± 1.4 (mean ± SD) ARTIs, while the placebo patients averaged 5.2 ± 1.5 ARTIs (-2.4 [3.3, -1.5] mean difference [CI 95 %] p < 0.001 by Student's t test). There were no significant correlations between the number of infections and the change in the IgG4 levels in either group (p > 0.05), nor were there differences in the number of infections between the patients with positive or negative changes in IgG4, nor were there differences in IgG4 levels in the patients grouped as those with ≤ 3 or > 3 infections in either group (p > 0.05 by Mann-Whitney U test).
The patients in the OM-85 group received 21,3 (median (upper, lower quartiles)) antibiotic treatments (prescribed by Dr. Del-Rio-Navarro and Dr. Avila-Castañón), while the patients in the placebo group had 32,5 antibiotic treatments (-2.0 [-3, -1] median difference [95 % CI] p < 0.001 by Mann-Whitney U test).
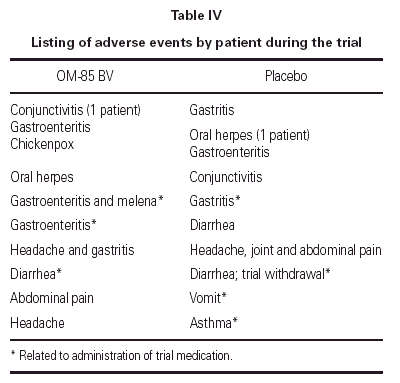
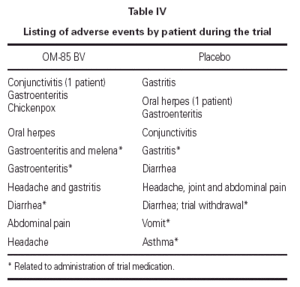
Eight patients in the OM-85 BV group presented 10 adverse events; only three were related to drug administration. Nine patients taking placebos had 10 adverse events; four were related to the administration of the placebo. One patient in the placebo group was withdrawn because diarrhea. See table IV.
DISCUSSION
IgG subclass deficiency is frequently associated with RARTIs. The reported prevalence of IgG subclass deficiency is up to 63 % in these patients1. In the present study we found that 49 out of 63 (78 %) patients with RARTIs had subnormal levels of IgG subclasses. Because OM-85 BV has induced clinical improvement in patients suffering RARTIs34,35, IgG or IgA deficiency36, and common variable immunodeficiency37, we had expected the correction of IgG subclass defects. In this study, OM-85 BV did reduce the number of ARTIs almost by 50 % as in previous trials33,35. Yet, in OM-85 BV patients, the only significant IgG change was the reduction of IgG4 levels. There was no increment in the total IgG concentration as reported in the previous trials27, but there was a trend to increase the IgG1 concentration, which is the IgG subclass with the higher concentration. There was also a trend for IgG3 reduction.
IgG4 production has been associated with IgE production, as they share part of the regulatory mechanisms42,43 and the presence of IgG4 to allergens preceded the development of IgE response to these allergens and the beginning of atopy and asthma44,45. IgG4 reduction may be ascribed to the downward regulation of Th2 responses. It has been demonstrated that OM-85 BV induces the reduction of IgE levels28,31, and recent studies have shown that immunostimulants shift Th2 responses to Th1 responses46,47.
Yet, the reduction of ARTI induced by OM-85 BV may be ascribed to other compensating mechanisms, such as the increase of secretory IgA levels. OM-85 BV induces the increase of secretory IgA27-29 and it has been postulated that secretory IgA may compensate the IgG subclass deficiencies48. On another other hand, the reduction of IgG4 may be due to the reduction in the number of ARTIs. Increases in IgG4 levels have been found during repetitive viral infections49.
As in this trial, as well as in previous trials32,33,35 OM-85 BV and placebo group presented gastrointestinal side effects, at least some of the cases of gastrointestinal side effects may due to the presence of lactose in the excipient.
The incidence, prevalence, and particularly the evolution of patients suffering from RARTIs in conjunction with subnormal levels of IgG subclasses require further characterization. Recently Lawton has described the difficulties of establishing a cutoff level for the diagnosis of IgG subclass deficiencies and pointed out that reductions in IgG subclasses in patients with recurrent infections are manifestations of an immunoregulatory disorder that also causes defects in the humoral responses to specific antigens rather than immunodeficiencies themselves50.
The effect of OM-85 BV on the IgG4 levels must be confirmed with further studies considering the evolution of the IgG subclasses at different times in larger samples of normal subjects and patients suffering from RARTIs. Additionally, the simultaneous effect of OM-85 BV on the immune response to polysaccharides must be evaluated, as well as the effect on IgE and secretory IgA levels. The present study demonstrated the clinical benefit of OM-85 BV on patients suffering RARTIs in conjunction with subnormal levels of IgG subclasses and it opened a new perspective in the research of the mechanism of action behind this clinical benefit.
ACKNOWLEDGMENTS
Dr. Arturo Berber was the medical manager for OM-85 BV in Mexico from 1995 to 2000. Química Knoll de México, BASF Pharma sponsored this study. Química Knoll de México, BASF Pharma commercialized OM-85 BV in Mexico from 1995 to 2001.