Asthma prevalence has been reported to be lower in rural areas compared to urban areas, although this has been inconsistent. This study aims to identify the influence of urban–rural residence on asthma prevalence in adolescents in the Republic of Macedonia and to investigate characteristics that may explain observed associations.
MethodsFollowing International Study of Asthma and Allergies in Childhood protocol, a national sample of Macedonian urban and rural dwelling adolescents (12–16 years) was recruited in 2006. Self-completed questionnaires were used to collect data on wheeze and asthma as well as personal, environmental and dietary characteristics. Following descriptive and multiple logistic regression analyses, a mediation analysis approach was performed to help explain observed associations.
ResultsA lower prevalence of current wheeze and ever-diagnosed asthma was observed in rural compared to urban dwelling adolescents (4.9% vs. 7.2% and 1.2% vs. 1.9%, respectively). After adjustment for potential confounders, the associations, although still protective, were not statistically significant (wheeze: OR=0.74, 95%CI=0.46–1.21; asthma: OR=0.97, 95%CI=0.38–2.46). The associations between urban–rural status with current wheeze and asthma were mediated by region of the country (wheeze 9%; asthma 19%) and by diet (>5% change for both wheeze and asthma). Having a dog resulted in a strengthening of the association between urban–rural status and current wheeze by 11.9%.
ConclusionsThe prevalence of asthma and wheeze was lower in rural dwelling Macedonian adolescents and the association was mediated by the region of the country with diet likely to be part of the reason for this mediating effect.
Wide variations in the prevalence of childhood asthma have been documented worldwide with more prevalent asthma symptoms in more affluent countries.1 ‘Western lifestyle’ and urbanisation have been suggested to explain the worldwide geographic variation in asthma prevalence.2 In support of this, urban–rural differences in asthma prevalence have been observed, with most comparative studies in adults and children having reported lower asthma prevalence in rural compared to urban areas,3–5 although some studies have not found such differences.6,7 Establishing the reasons for the protective role of rural residence on asthma prevalence is very important for aetiologic understanding leading to future prevention and identification of treatment strategies, as the global asthma burden continues to rise.8
A number of reasons could explain the urban–rural differences in asthma prevalence, including differences in diagnosing patterns, environmental exposures, and behaviours. While microbial exposures from animals or unpasteurised milk in rural areas have been highlighted as potential protective factors,9,10 the aforementioned reasons should be considered as part of the Westernisation and urbanisation package.
Compared to worldwide prevalence rates of asthma, the Republic of Macedonia appears to have a moderately low prevalence of current wheeze1 and a low prevalence of ever-diagnosed asthma.2 Given this fact, the paucity of information on asthma in adolescents, and the need to investigate the underlying reasons for the apparent association between urban–rural status and asthma, the objective of this study was to determine if there was an association between wheeze and asthma with rural dwelling among a national sample of Macedonian adolescents and, if so, to investigate the characteristics that may explain this association. As part of this process, we sought to profile and compare urban and rural dwelling Macedonian adolescents.
Subjects and methodsStudy population and proceduresThis was a cross-sectional study conducted in 2005–2006 in eight cities and their adjacent villages in the Republic of Macedonia. The study was approved by The Ethics Committee at the Medical Faculty and The Ministry of Education and Science, Skopje, the Republic of Macedonia.
Selection of participants and data collection were performed following the International Study of Asthma and Allergies in Childhood (ISAAC) Phase 3 protocol, which has been published elsewhere.11,12 In brief, following random selection of primary schools, informed consent was obtained from parents and 12–16-year-old children to self-complete the standardised ISAAC Phase 3 written questionnaires on asthma, rhinitis, eczema and related factors. This was completed at the school. No additional questions were included in the original form of the questionnaires, with the exception of the questions regarding the close relative's history of allergic diseases.
The larger cities and surrounding rural areas included in the study consisted of Skopje (population=578,144), Veles (population=55,108) and Tetovo (population=86,580), with the latter two cities approximately 50km from Skopje and all three cities located in the northern part of the country as well as Ohrid (population=55,749), located 200km from Skopje in the western part of the country and characterised by a similar level of affluence and urbanisation as the cities in the northern part of the country. The other four cities involved in the study (Berovo, Pehcevo, Delcevo, Makedonska Kamenica) are smaller cities (populations<15,000) close to each other and located in the less developed eastern part of the country, approximately 200km from Skopje.
Operational definitionsThe health outcomes of interest in this study were current wheeze and ever-diagnosed asthma defined as wheeze in the past 12 months and ever having had asthma diagnosed by a doctor, respectively.1,12 The primary exposure of interest was geographic location of dwelling (urban–rural status). Respondents from the eight cities in the study were classified as urban dwelling while those living in the nearby villages were classified as rural dwelling respondents. Other personal, environmental and dietary characteristics were considered as independent factors and confounders. Their descriptions follow.
Personal characteristics: Socio-demographic characteristics were considered, including sex, age, nationality (Macedonian and non-Macedonian) and region of the country (North/Western Macedonia and Eastern Macedonia). Mother's educational level was classified as primary, secondary and tertiary (university) education. Additionally, maternal and paternal history of allergic diseases (yes or no responses) and respondent's birth order were considered.
Overweight/obesity status. According to the ISAAC protocol, self-reported weight and height were used for the calculation of body mass index (BMI) of each respondent as weight (kg)/height (m)2. In some cases, the subjects did not know their height and weight. In these cases, height and weight were objectively measured. The international cut-off points for BMI for overweight and obesity, defined to pass through a BMI of 25kg/m2 for overweight and 30kg/m2 for obesity at age 18, were used.13 As only 1.5% of the respondents were obese, they were included in the overweight group.
Physical activity participation was based on the response to the question “How many times a week do you engage in vigorous physical activity long enough to make your breathe hard?” The respondents were categorised into either never/occasionally, once/twice per week or three/more times a week physical activity categories.
Television (TV) watching/PC playing time was defined based on the question “During a normal week, how many hours a day (24h) do you watch television or use a PC for playing games?” Responses were classified as less than 1h, 1h but <3h, 3h but <5h, and 5h or more.
Responses about average Paracetamol use in the past year were categorised into never, at least once a year and at least once a month categories.
Environmental characteristics: Maternal and paternal smoking habits anytime in the past year, cat and dog ownership in the past year, fuel usually used for home cooking (electricity, gas, open fires) and fuel usually used for home heating (electricity, wood) were all assessed on the basis of yes-no responses.
Frequency of truck passing through the residential street was defined based on the question “How often do trucks pass through the street where you live, on weekdays?” and was categorised into never/seldom and frequently/almost whole day categories.
Dietary characteristics: Participants were asked how often during the past 12 months they usually ate or drank different dietary products (meat, fish, fruit, vegetables, pulses, cereal, pasta, rice, margarine, milk, fast food) and responses for each food type were classified into low (never or occasionally), moderate (once or twice per week) and high (three or more times a week) categories.
Statistical analysisStatistical analysis was completed using SPSS 20. Following descriptive analyses, comparisons between urban and rural dwelling respondents with regard to personal, environmental, and dietary characteristics were completed using the chi-squared tests for categorical variables and independent samples t-tests for continuous measures.
The next series of analyses considered the outcomes of current wheeze and asthma. Multiple logistic regression was completed after selecting variables following the protocol of Hosmer and Lemeshow.14 Variables were selected to the final model based on statistical significance, clinical or biological importance based on the scientific literature, and the impact that the removal of a variable had on the beta coefficients of the variables remaining in the model. As urban–rural status was the primary exposure of interest, it was retained in the final models. The final model fitted for each outcome (current wheeze and asthma) included variables selected for either outcome to ensure consistent interpretations of results between models. The strength of associations was assessed by the odds ratio (OR) and 95% confidence intervals (CI).
A mediation analysis approach was performed for each outcome. This was similar to those from previously completed health studies.15 Initially, a base model was fitted which included urban–rural status and each of the variables selected based on confounding assessments. Next, one variable at a time was added to the baseline model to assess the impact it had on the association between urban–rural status and each outcome. For mediation to occur, a statistically significant association between the primary exposure (urban–rural status) and the outcome must be smaller and no longer statistically significant when the mediator is included.15 The percentage change for the association of urban–rural status from the adjusted baseline model was calculated for each variable or group of variables added. A change of 5% or more was considered an important difference.
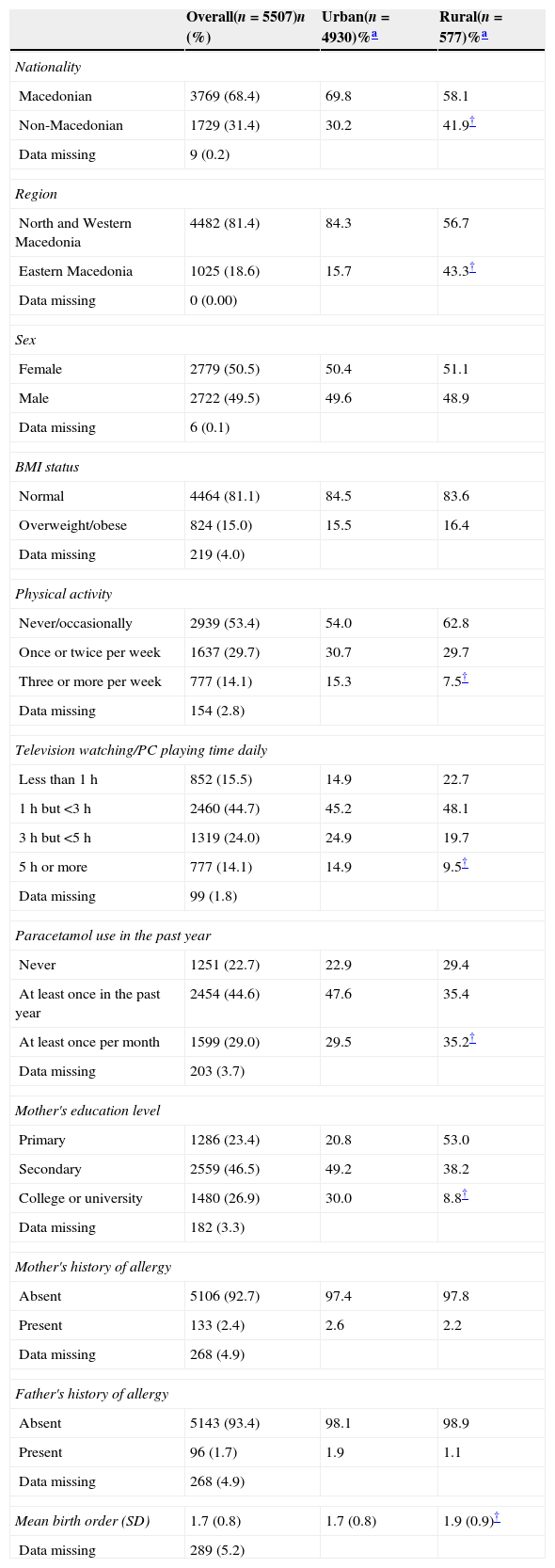
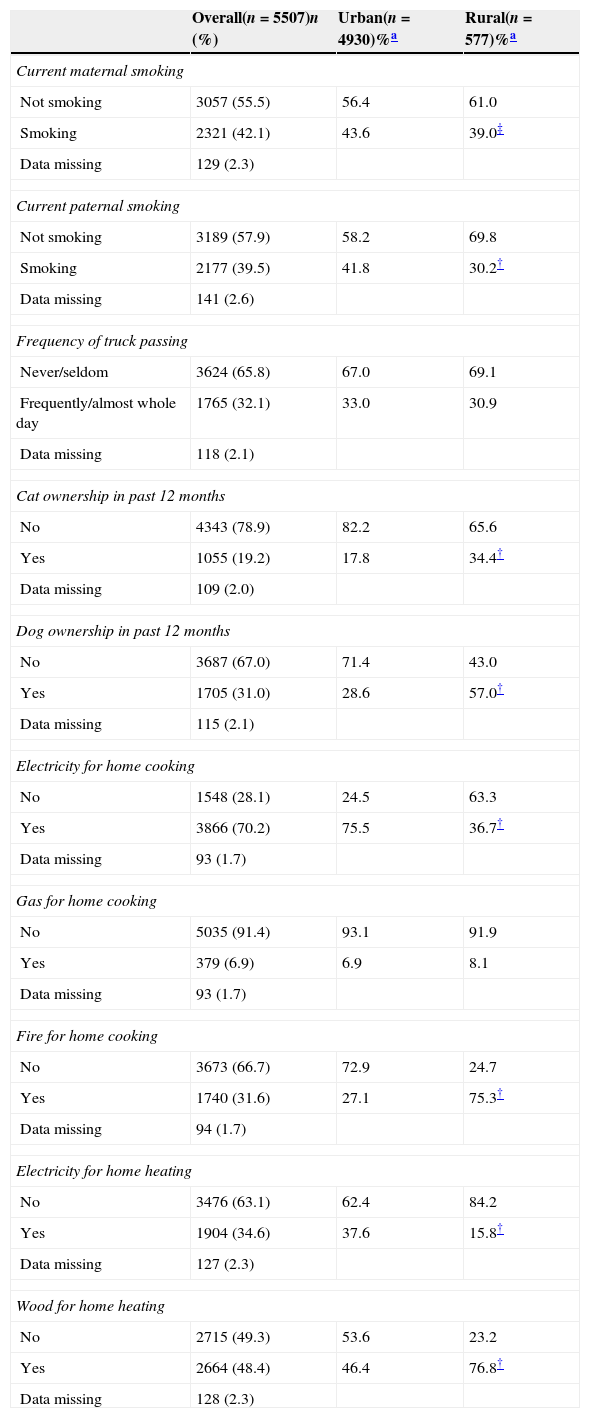
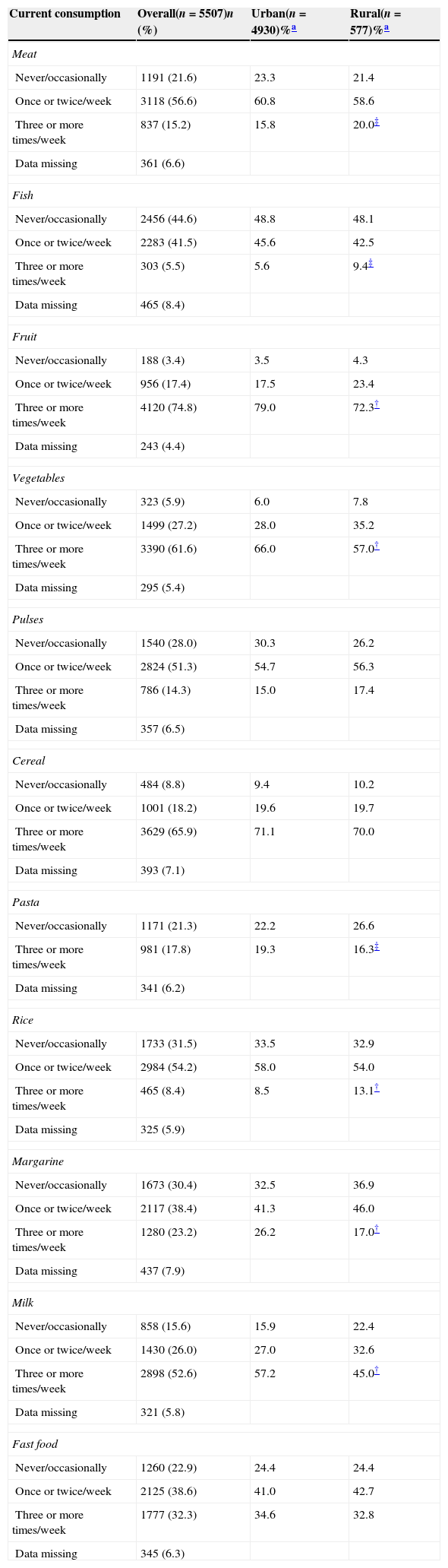
ResultsIn total, 5507 adolescents took part in this study (90.3% participation rate). Of these, 577 (10.5%) resided in rural areas. The study population was evenly distributed by sex (50.5% female) with most adolescents residing in North and Western Macedonia (81.4%) and being of Macedonian ethnic background (68.4%). With regard to personal characteristics, compared to urban areas, a higher proportion of adolescents from rural areas were non-Macedonian, from Eastern Macedonia, and had used Paracetamol at least once per month in the past year but a lower proportion had physical activity three or more times per week, had 5h or more per day of screen time, and had a mother with university education (Table 1). With regard to environmental exposures, those from rural areas were less likely to have a mother or father who smoked, use electricity for cooking or home heating but had a higher proportion of adolescents reporting a cat or dog ownership, using fire for cooking or wood for home heating compared to urban dwellers (Table 2). Finally, there were differences between urban and rural dwellers regarding dietary characteristics where rural dwellers ate higher amounts of meat, fish, and rice but lower amounts of fruits, vegetables, pasta, margarine, and milk (Table 3).
Distribution of personal characteristics by urban–rural status.
| Overall(n=5507)n (%) | Urban(n=4930)%a | Rural(n=577)%a | |
|---|---|---|---|
| Nationality | |||
| Macedonian | 3769 (68.4) | 69.8 | 58.1 |
| Non-Macedonian | 1729 (31.4) | 30.2 | 41.9† |
| Data missing | 9 (0.2) | ||
| Region | |||
| North and Western Macedonia | 4482 (81.4) | 84.3 | 56.7 |
| Eastern Macedonia | 1025 (18.6) | 15.7 | 43.3† |
| Data missing | 0 (0.00) | ||
| Sex | |||
| Female | 2779 (50.5) | 50.4 | 51.1 |
| Male | 2722 (49.5) | 49.6 | 48.9 |
| Data missing | 6 (0.1) | ||
| BMI status | |||
| Normal | 4464 (81.1) | 84.5 | 83.6 |
| Overweight/obese | 824 (15.0) | 15.5 | 16.4 |
| Data missing | 219 (4.0) | ||
| Physical activity | |||
| Never/occasionally | 2939 (53.4) | 54.0 | 62.8 |
| Once or twice per week | 1637 (29.7) | 30.7 | 29.7 |
| Three or more per week | 777 (14.1) | 15.3 | 7.5† |
| Data missing | 154 (2.8) | ||
| Television watching/PC playing time daily | |||
| Less than 1h | 852 (15.5) | 14.9 | 22.7 |
| 1h but <3h | 2460 (44.7) | 45.2 | 48.1 |
| 3h but <5h | 1319 (24.0) | 24.9 | 19.7 |
| 5h or more | 777 (14.1) | 14.9 | 9.5† |
| Data missing | 99 (1.8) | ||
| Paracetamol use in the past year | |||
| Never | 1251 (22.7) | 22.9 | 29.4 |
| At least once in the past year | 2454 (44.6) | 47.6 | 35.4 |
| At least once per month | 1599 (29.0) | 29.5 | 35.2† |
| Data missing | 203 (3.7) | ||
| Mother's education level | |||
| Primary | 1286 (23.4) | 20.8 | 53.0 |
| Secondary | 2559 (46.5) | 49.2 | 38.2 |
| College or university | 1480 (26.9) | 30.0 | 8.8† |
| Data missing | 182 (3.3) | ||
| Mother's history of allergy | |||
| Absent | 5106 (92.7) | 97.4 | 97.8 |
| Present | 133 (2.4) | 2.6 | 2.2 |
| Data missing | 268 (4.9) | ||
| Father's history of allergy | |||
| Absent | 5143 (93.4) | 98.1 | 98.9 |
| Present | 96 (1.7) | 1.9 | 1.1 |
| Data missing | 268 (4.9) | ||
| Mean birth order (SD) | 1.7 (0.8) | 1.7 (0.8) | 1.9 (0.9)† |
| Data missing | 289 (5.2) | ||
Distribution of environmental characteristics by urban–rural status.
| Overall(n=5507)n (%) | Urban(n=4930)%a | Rural(n=577)%a | |
|---|---|---|---|
| Current maternal smoking | |||
| Not smoking | 3057 (55.5) | 56.4 | 61.0 |
| Smoking | 2321 (42.1) | 43.6 | 39.0‡ |
| Data missing | 129 (2.3) | ||
| Current paternal smoking | |||
| Not smoking | 3189 (57.9) | 58.2 | 69.8 |
| Smoking | 2177 (39.5) | 41.8 | 30.2† |
| Data missing | 141 (2.6) | ||
| Frequency of truck passing | |||
| Never/seldom | 3624 (65.8) | 67.0 | 69.1 |
| Frequently/almost whole day | 1765 (32.1) | 33.0 | 30.9 |
| Data missing | 118 (2.1) | ||
| Cat ownership in past 12 months | |||
| No | 4343 (78.9) | 82.2 | 65.6 |
| Yes | 1055 (19.2) | 17.8 | 34.4† |
| Data missing | 109 (2.0) | ||
| Dog ownership in past 12 months | |||
| No | 3687 (67.0) | 71.4 | 43.0 |
| Yes | 1705 (31.0) | 28.6 | 57.0† |
| Data missing | 115 (2.1) | ||
| Electricity for home cooking | |||
| No | 1548 (28.1) | 24.5 | 63.3 |
| Yes | 3866 (70.2) | 75.5 | 36.7† |
| Data missing | 93 (1.7) | ||
| Gas for home cooking | |||
| No | 5035 (91.4) | 93.1 | 91.9 |
| Yes | 379 (6.9) | 6.9 | 8.1 |
| Data missing | 93 (1.7) | ||
| Fire for home cooking | |||
| No | 3673 (66.7) | 72.9 | 24.7 |
| Yes | 1740 (31.6) | 27.1 | 75.3† |
| Data missing | 94 (1.7) | ||
| Electricity for home heating | |||
| No | 3476 (63.1) | 62.4 | 84.2 |
| Yes | 1904 (34.6) | 37.6 | 15.8† |
| Data missing | 127 (2.3) | ||
| Wood for home heating | |||
| No | 2715 (49.3) | 53.6 | 23.2 |
| Yes | 2664 (48.4) | 46.4 | 76.8† |
| Data missing | 128 (2.3) | ||
Distribution of dietary characteristics by urban–rural status.
| Current consumption | Overall(n=5507)n (%) | Urban(n=4930)%a | Rural(n=577)%a |
|---|---|---|---|
| Meat | |||
| Never/occasionally | 1191 (21.6) | 23.3 | 21.4 |
| Once or twice/week | 3118 (56.6) | 60.8 | 58.6 |
| Three or more times/week | 837 (15.2) | 15.8 | 20.0‡ |
| Data missing | 361 (6.6) | ||
| Fish | |||
| Never/occasionally | 2456 (44.6) | 48.8 | 48.1 |
| Once or twice/week | 2283 (41.5) | 45.6 | 42.5 |
| Three or more times/week | 303 (5.5) | 5.6 | 9.4‡ |
| Data missing | 465 (8.4) | ||
| Fruit | |||
| Never/occasionally | 188 (3.4) | 3.5 | 4.3 |
| Once or twice/week | 956 (17.4) | 17.5 | 23.4 |
| Three or more times/week | 4120 (74.8) | 79.0 | 72.3† |
| Data missing | 243 (4.4) | ||
| Vegetables | |||
| Never/occasionally | 323 (5.9) | 6.0 | 7.8 |
| Once or twice/week | 1499 (27.2) | 28.0 | 35.2 |
| Three or more times/week | 3390 (61.6) | 66.0 | 57.0† |
| Data missing | 295 (5.4) | ||
| Pulses | |||
| Never/occasionally | 1540 (28.0) | 30.3 | 26.2 |
| Once or twice/week | 2824 (51.3) | 54.7 | 56.3 |
| Three or more times/week | 786 (14.3) | 15.0 | 17.4 |
| Data missing | 357 (6.5) | ||
| Cereal | |||
| Never/occasionally | 484 (8.8) | 9.4 | 10.2 |
| Once or twice/week | 1001 (18.2) | 19.6 | 19.7 |
| Three or more times/week | 3629 (65.9) | 71.1 | 70.0 |
| Data missing | 393 (7.1) | ||
| Pasta | |||
| Never/occasionally | 1171 (21.3) | 22.2 | 26.6 |
| Three or more times/week | 981 (17.8) | 19.3 | 16.3‡ |
| Data missing | 341 (6.2) | ||
| Rice | |||
| Never/occasionally | 1733 (31.5) | 33.5 | 32.9 |
| Once or twice/week | 2984 (54.2) | 58.0 | 54.0 |
| Three or more times/week | 465 (8.4) | 8.5 | 13.1† |
| Data missing | 325 (5.9) | ||
| Margarine | |||
| Never/occasionally | 1673 (30.4) | 32.5 | 36.9 |
| Once or twice/week | 2117 (38.4) | 41.3 | 46.0 |
| Three or more times/week | 1280 (23.2) | 26.2 | 17.0† |
| Data missing | 437 (7.9) | ||
| Milk | |||
| Never/occasionally | 858 (15.6) | 15.9 | 22.4 |
| Once or twice/week | 1430 (26.0) | 27.0 | 32.6 |
| Three or more times/week | 2898 (52.6) | 57.2 | 45.0† |
| Data missing | 321 (5.8) | ||
| Fast food | |||
| Never/occasionally | 1260 (22.9) | 24.4 | 24.4 |
| Once or twice/week | 2125 (38.6) | 41.0 | 42.7 |
| Three or more times/week | 1777 (32.3) | 34.6 | 32.8 |
| Data missing | 345 (6.3) | ||
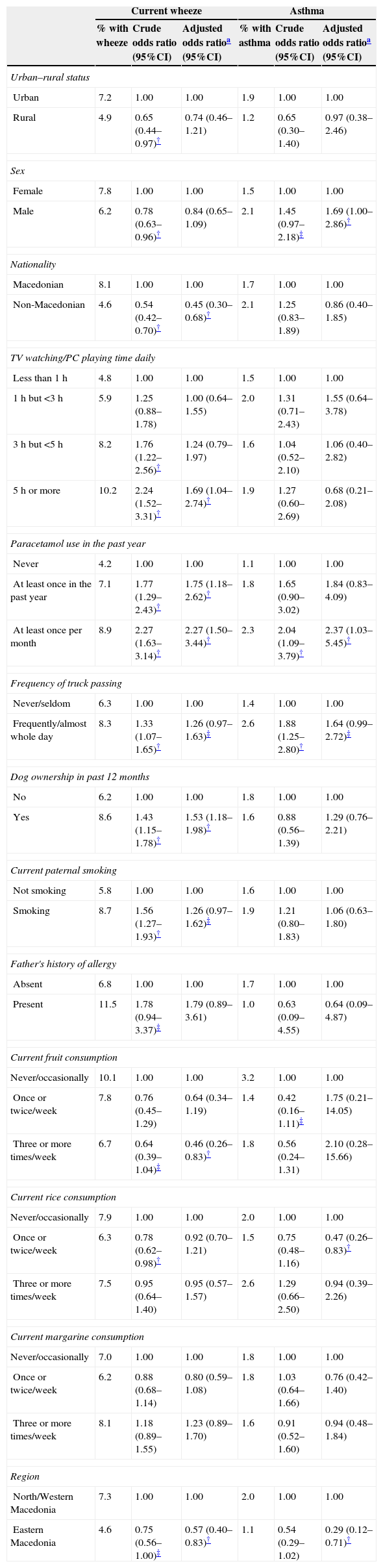
The prevalence of current wheeze was lower in rural dwellers compared to urban dwellers (4.9% vs. 7.2%, p=0.03) and this was also true for the prevalence of asthma (1.2% vs. 1.9%, p=0.26). After adjustment for potential confounders, the associations remained protective but were weaker and no longer statistically significant for current wheeze (Table 4).
Prevalence and results from logistic regression analyses with current wheeze and asthma as the outcomes.
| Current wheeze | Asthma | |||||
|---|---|---|---|---|---|---|
| % with wheeze | Crude odds ratio (95%CI) | Adjusted odds ratioa (95%CI) | % with asthma | Crude odds ratio (95%CI) | Adjusted odds ratioa (95%CI) | |
| Urban–rural status | ||||||
| Urban | 7.2 | 1.00 | 1.00 | 1.9 | 1.00 | 1.00 |
| Rural | 4.9 | 0.65 (0.44–0.97)† | 0.74 (0.46–1.21) | 1.2 | 0.65 (0.30–1.40) | 0.97 (0.38–2.46) |
| Sex | ||||||
| Female | 7.8 | 1.00 | 1.00 | 1.5 | 1.00 | 1.00 |
| Male | 6.2 | 0.78 (0.63–0.96)† | 0.84 (0.65–1.09) | 2.1 | 1.45 (0.97–2.18)‡ | 1.69 (1.00–2.86)† |
| Nationality | ||||||
| Macedonian | 8.1 | 1.00 | 1.00 | 1.7 | 1.00 | 1.00 |
| Non-Macedonian | 4.6 | 0.54 (0.42–0.70)† | 0.45 (0.30–0.68)† | 2.1 | 1.25 (0.83–1.89) | 0.86 (0.40–1.85) |
| TV watching/PC playing time daily | ||||||
| Less than 1h | 4.8 | 1.00 | 1.00 | 1.5 | 1.00 | 1.00 |
| 1h but <3h | 5.9 | 1.25 (0.88–1.78) | 1.00 (0.64–1.55) | 2.0 | 1.31 (0.71–2.43) | 1.55 (0.64–3.78) |
| 3h but <5h | 8.2 | 1.76 (1.22–2.56)† | 1.24 (0.79–1.97) | 1.6 | 1.04 (0.52–2.10) | 1.06 (0.40–2.82) |
| 5h or more | 10.2 | 2.24 (1.52–3.31)† | 1.69 (1.04–2.74)† | 1.9 | 1.27 (0.60–2.69) | 0.68 (0.21–2.08) |
| Paracetamol use in the past year | ||||||
| Never | 4.2 | 1.00 | 1.00 | 1.1 | 1.00 | 1.00 |
| At least once in the past year | 7.1 | 1.77 (1.29–2.43)† | 1.75 (1.18–2.62)† | 1.8 | 1.65 (0.90–3.02) | 1.84 (0.83–4.09) |
| At least once per month | 8.9 | 2.27 (1.63–3.14)† | 2.27 (1.50–3.44)† | 2.3 | 2.04 (1.09–3.79)† | 2.37 (1.03–5.45)† |
| Frequency of truck passing | ||||||
| Never/seldom | 6.3 | 1.00 | 1.00 | 1.4 | 1.00 | 1.00 |
| Frequently/almost whole day | 8.3 | 1.33 (1.07–1.65)† | 1.26 (0.97–1.63)‡ | 2.6 | 1.88 (1.25–2.80)† | 1.64 (0.99–2.72)‡ |
| Dog ownership in past 12 months | ||||||
| No | 6.2 | 1.00 | 1.00 | 1.8 | 1.00 | 1.00 |
| Yes | 8.6 | 1.43 (1.15–1.78)† | 1.53 (1.18–1.98)† | 1.6 | 0.88 (0.56–1.39) | 1.29 (0.76–2.21) |
| Current paternal smoking | ||||||
| Not smoking | 5.8 | 1.00 | 1.00 | 1.6 | 1.00 | 1.00 |
| Smoking | 8.7 | 1.56 (1.27–1.93)† | 1.26 (0.97–1.62)‡ | 1.9 | 1.21 (0.80–1.83) | 1.06 (0.63–1.80) |
| Father's history of allergy | ||||||
| Absent | 6.8 | 1.00 | 1.00 | 1.7 | 1.00 | 1.00 |
| Present | 11.5 | 1.78 (0.94–3.37)‡ | 1.79 (0.89–3.61) | 1.0 | 0.63 (0.09–4.55) | 0.64 (0.09–4.87) |
| Current fruit consumption | ||||||
| Never/occasionally | 10.1 | 1.00 | 1.00 | 3.2 | 1.00 | 1.00 |
| Once or twice/week | 7.8 | 0.76 (0.45–1.29) | 0.64 (0.34–1.19) | 1.4 | 0.42 (0.16–1.11)‡ | 1.75 (0.21–14.05) |
| Three or more times/week | 6.7 | 0.64 (0.39–1.04)‡ | 0.46 (0.26–0.83)† | 1.8 | 0.56 (0.24–1.31) | 2.10 (0.28–15.66) |
| Current rice consumption | ||||||
| Never/occasionally | 7.9 | 1.00 | 1.00 | 2.0 | 1.00 | 1.00 |
| Once or twice/week | 6.3 | 0.78 (0.62–0.98)† | 0.92 (0.70–1.21) | 1.5 | 0.75 (0.48–1.16) | 0.47 (0.26–0.83)† |
| Three or more times/week | 7.5 | 0.95 (0.64–1.40) | 0.95 (0.57–1.57) | 2.6 | 1.29 (0.66–2.50) | 0.94 (0.39–2.26) |
| Current margarine consumption | ||||||
| Never/occasionally | 7.0 | 1.00 | 1.00 | 1.8 | 1.00 | 1.00 |
| Once or twice/week | 6.2 | 0.88 (0.68–1.14) | 0.80 (0.59–1.08) | 1.8 | 1.03 (0.64–1.66) | 0.76 (0.42–1.40) |
| Three or more times/week | 8.1 | 1.18 (0.89–1.55) | 1.23 (0.89–1.70) | 1.6 | 0.91 (0.52–1.60) | 0.94 (0.48–1.84) |
| Region | ||||||
| North/Western Macedonia | 7.3 | 1.00 | 1.00 | 2.0 | 1.00 | 1.00 |
| Eastern Macedonia | 4.6 | 0.75 (0.56–1.00)‡ | 0.57 (0.40–0.83)† | 1.1 | 0.54 (0.29–1.02) | 0.29 (0.12–0.71)† |
When considering current wheeze as the outcome (Table 4), inverse associations were seen with being non-Macedonian, eating fruit three or more times per week and living in Eastern Macedonia. Watching television or playing PC games more than 5h per day, Paracetamol use and having a dog in the home increased the risk of having current wheeze. Dose–response relationships were observed with fruit consumption, screen time and Paracetamol use. There were also trends (p<0.10) of an increased risk of current wheeze associated with a higher frequency of truck passing and current paternal smoking.
When considering asthma as the outcome (Table 4), in adjusted analyses there was an increased risk of asthma associated with being male and higher Paracetamol use and inverse associations with rice when it was consumed 1–2 times per week and living in Eastern Macedonia. A dose–response relationship was observed for Paracetamol use. Again, there was a trend (p<0.10) towards an increased risk of asthma associated with a higher frequency of truck passing.
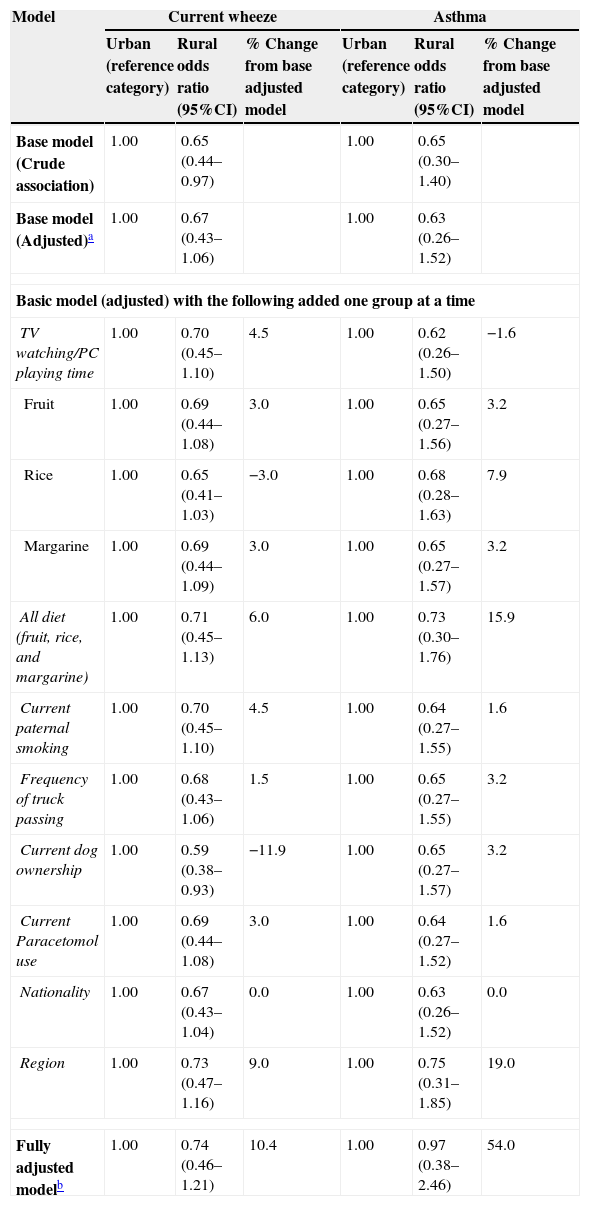
Table 5 presents the results of our mediation analysis approach. The association between urban–rural status and current wheeze increased (i.e. became weaker as it approached the null value) by 5% or greater when region or all three dietary variables were included. Similarly, the association between urban–rural status and asthma changed by more than 5% when region, rice, or all three dietary variables were added to the model. The addition of having a dog resulted in a strengthening of the association between urban–rural status and current wheeze by 11.9%. For both current wheeze and asthma, the largest change towards the null came with the addition of region (9% and 19%, respectively).
Adjusted association between urban–rural status and current wheeze and asthma then addition of each factor to explore potential mediation.
| Model | Current wheeze | Asthma | ||||
|---|---|---|---|---|---|---|
| Urban (reference category) | Rural odds ratio (95%CI) | % Change from base adjusted model | Urban (reference category) | Rural odds ratio (95%CI) | % Change from base adjusted model | |
| Base model (Crude association) | 1.00 | 0.65 (0.44–0.97) | 1.00 | 0.65 (0.30–1.40) | ||
| Base model (Adjusted)a | 1.00 | 0.67 (0.43–1.06) | 1.00 | 0.63 (0.26–1.52) | ||
| Basic model (adjusted) with the following added one group at a time | ||||||
| TV watching/PC playing time | 1.00 | 0.70 (0.45–1.10) | 4.5 | 1.00 | 0.62 (0.26–1.50) | −1.6 |
| Fruit | 1.00 | 0.69 (0.44–1.08) | 3.0 | 1.00 | 0.65 (0.27–1.56) | 3.2 |
| Rice | 1.00 | 0.65 (0.41–1.03) | −3.0 | 1.00 | 0.68 (0.28–1.63) | 7.9 |
| Margarine | 1.00 | 0.69 (0.44–1.09) | 3.0 | 1.00 | 0.65 (0.27–1.57) | 3.2 |
| All diet (fruit, rice, and margarine) | 1.00 | 0.71 (0.45–1.13) | 6.0 | 1.00 | 0.73 (0.30–1.76) | 15.9 |
| Current paternal smoking | 1.00 | 0.70 (0.45–1.10) | 4.5 | 1.00 | 0.64 (0.27–1.55) | 1.6 |
| Frequency of truck passing | 1.00 | 0.68 (0.43–1.06) | 1.5 | 1.00 | 0.65 (0.27–1.55) | 3.2 |
| Current dog ownership | 1.00 | 0.59 (0.38–0.93) | −11.9 | 1.00 | 0.65 (0.27–1.57) | 3.2 |
| Current Paracetomol use | 1.00 | 0.69 (0.44–1.08) | 3.0 | 1.00 | 0.64 (0.27–1.52) | 1.6 |
| Nationality | 1.00 | 0.67 (0.43–1.04) | 0.0 | 1.00 | 0.63 (0.26–1.52) | 0.0 |
| Region | 1.00 | 0.73 (0.47–1.16) | 9.0 | 1.00 | 0.75 (0.31–1.85) | 19.0 |
| Fully adjusted modelb | 1.00 | 0.74 (0.46–1.21) | 10.4 | 1.00 | 0.97 (0.38–2.46) | 54.0 |
Adjusted for urban–rural status, age, sex, physical activity, fish consumption, fast food consumption, gas cooking, mother's education level, and current maternal smoking.
Adjusted for urban–rural status, age, sex, physical activity, television watching/PC playing time, paracetomol use, frequency of truck passing, dog ownership in the past 12 months, current maternal smoking, current paternal smoking, father's history of allergy, fish consumption, fruit consumption, rice consumption, margarine consumption, fast food consumption, gas cooking, and mother's education level.
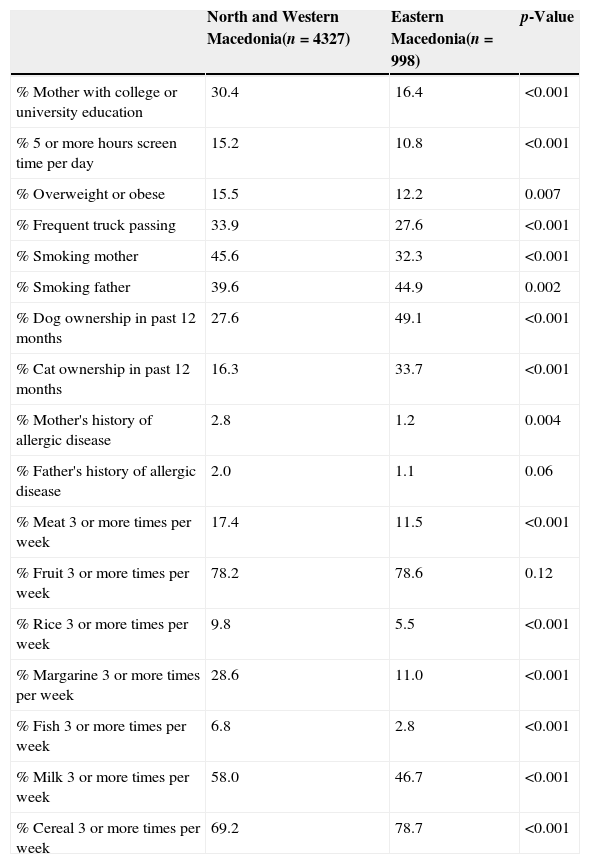
In order to better understand the differences between regions within Macedonia as a mediating factor in the association between urban–rural status and current wheeze and asthma, we completed a comparison of personal, health behaviour, dietary, and environmental characteristics between regions (Table 6). We found that there were significant differences between regions that suggest lower education levels, screen time, air pollution, and overweight/obesity and more dog and cat ownership in Eastern Macedonia compared to the north and west region. We also found differences in the dietary habits between regions. Finally, when considering a ratio of current wheeze-diagnosed asthma, we found that the ratio was lower in the north and west region (3.7:1) compared to the eastern region (5.1:1) which may suggest differences in diagnosing patterns.
Results of a descriptive comparison between North and Western Macedonia with Eastern Macedonia on personal, health behaviour, dietary, and environmental characteristics.
| North and Western Macedonia(n=4327) | Eastern Macedonia(n=998) | p-Value | |
|---|---|---|---|
| % Mother with college or university education | 30.4 | 16.4 | <0.001 |
| % 5 or more hours screen time per day | 15.2 | 10.8 | <0.001 |
| % Overweight or obese | 15.5 | 12.2 | 0.007 |
| % Frequent truck passing | 33.9 | 27.6 | <0.001 |
| % Smoking mother | 45.6 | 32.3 | <0.001 |
| % Smoking father | 39.6 | 44.9 | 0.002 |
| % Dog ownership in past 12 months | 27.6 | 49.1 | <0.001 |
| % Cat ownership in past 12 months | 16.3 | 33.7 | <0.001 |
| % Mother's history of allergic disease | 2.8 | 1.2 | 0.004 |
| % Father's history of allergic disease | 2.0 | 1.1 | 0.06 |
| % Meat 3 or more times per week | 17.4 | 11.5 | <0.001 |
| % Fruit 3 or more times per week | 78.2 | 78.6 | 0.12 |
| % Rice 3 or more times per week | 9.8 | 5.5 | <0.001 |
| % Margarine 3 or more times per week | 28.6 | 11.0 | <0.001 |
| % Fish 3 or more times per week | 6.8 | 2.8 | <0.001 |
| % Milk 3 or more times per week | 58.0 | 46.7 | <0.001 |
| % Cereal 3 or more times per week | 69.2 | 78.7 | <0.001 |
A lower prevalence of wheeze and asthma in rural dwelling Macedonian young adolescents compared to urban ones was observed with consistent results between analyses using the two outcomes. Statistical significance was occasionally lost due to differences in the strength of association between asthma or wheeze or to the lack of statistical power when asthma was the outcome. Given the higher overall prevalence of current wheeze compared to that of ever-diagnosed asthma and the general consistency in the related results under-diagnosis of asthma in early adolescence in our country can be inferred. For epidemiological research, asthma symptoms seem to be more reliable as the diagnosis of asthma in population-based studies is challenging, especially in developing countries where under-diagnosis of asthma is present.1
Our findings concur with previous research worldwide, demonstrating a protective effect of rural dwelling on asthma and wheeze. Sole et al.,16 analysing ISAAC Phase 3 data in 13–14 year-old Brazilian adolescents, reported higher prevalence rates of asthma-related symptoms and asthma diagnosis among adolescents living in urban compared to those living in rural areas. In a Canadian study involving young adolescents, a lower risk of current asthma was associated with rural and non-metro-adjacent dwelling in comparison to metro region dwelling.17 The compared results from two cross-sectional studies conducted in 7–8-year-old children living in urban and rural areas of Cyprus18 have shown lower prevalence rates of current wheeze and lifetime asthma in rural dwellers in 2000 and non-significantly different related prevalence rates between the both groups of children in 2008. Rises in current wheeze and asthma prevalence rates have been generally observed in both the urban and rural areas, but these consistently appeared more pronounced in the rural areas, indicating possible recent environmental and lifestyle changes there.
In contrast, Pesek et al.19 found a similar prevalence of diagnosed asthma in urban and rural children in Arkansas, with higher asthma morbidity in the previous two years in the rural group. In a Greek study that enrolled children aged 8–10 years, reported asthma and wheeze did not differ between urban and rural areas, whereas cough was more prevalent in the urban area. However, long-term exposure to an urban environment was found to be associated with sub-clinical airway narrowing.20
The results of the mediation analysis in the present study suggested that the region of Macedonia mediated, at least to some degree, the association between urban–rural status and current wheeze or asthma and that diet might be part of the reason for this mediating effect.
The comparison of personal, health behaviour, dietary, and environmental characteristics between regions within Macedonia pointed out many differences between Eastern Macedonia and North/Western Macedonia with similar differences seen between urban and rural areas. As a higher proportion of adolescents from rural areas were from Eastern Macedonia, the same factors may contribute to the lower prevalence of wheeze and asthma in rural areas. Also, under-diagnosis of asthma was more prevalent in the eastern region and it could not be completely ruled out as a possible reason for the lower asthma prevalence in this region and in rural dwelling adolescents; however current wheeze was also considered as an outcome showing lower prevalence by region and urban–rural status.
Diet showed different patterns in rural and urban dwellers with some associations seen with asthma and wheeze. “Pro-Mediterranean” foods rich in antioxidants, cis-monounsaturated fatty acids, and dietary fibre (fruit, vegetables, rice, fish, pulses, cereals, potatoes) have been postulated to act protectively while other food types (meat, fast food, trans-fatty acids in dairy products and margarine) to have an aggravating effect on asthma.21–23 In an earlier Canadian study, in contrast to our results, the association between asthma and an urban–rural gradient was not mediated by diet.17
The mediation analysis approach in the present study also suggested that current dog ownership strengthened the association between urban–rural status and current wheeze. The relation between exposure to cats and dogs and childhood asthma is complicated and the reported exposure–response relationships can be contradictory. Exposure to pets may increase the risk of developing allergic diseases in sensitised children through allergen production. Asthma symptoms may also be related to non-allergic mechanisms such as inflammation caused by endotoxin when encountered as part of a pathogen. However, development of tolerance to early-life pet allergen exposure or immune system modulation by endotoxin can explain the reported protective role of pet exposure on asthma.24 In the airways endotoxin may induce the production of inflammatory cytokines in the non-allergic Th1 immune pathway.25 There are inconsistent reports from epidemiological studies examining the role of endotoxin, linked to the presence of animals in home and farm living, and wheeze and asthma in childhood.25–27 While endotoxin exposure in early life and in farm residents appears to have a protective effect on asthma, there is evidence supporting its association with asthma severity and wheeze in schoolchildren.25
In addition, several modifiable health behaviours (screen time, Paracetamol use, current dog ownership and fruit consumption) were associated with current wheeze or asthma or both, irrespective of the location of residence and after adjustment for potential confounders. Asthma was documented to be inversely associated also with rice consumption 1–2 times per week, but not with more frequent consumption. This might be due to a poor diet where rice is consumed in great amounts instead of other healthy food. In general, lack of exercise and sedentary behaviours are associated with obesity and the latter with asthma, although there are complex relationships between each of them and asthma.28 In the last decade prenatal and postnatal Paracetamol exposure has been consistently reported as a risk factor for development and/or maintenance of asthma, with oxidant-induced airways inflammation and enhanced Th2 allergic immune responses as the main proposed underlying mechanisms.29,30
In the present study sex showed opposite associations with male respondents at increased risk for ever-diagnosed asthma and female respondents at increased risk for current wheeze. It is well known that in children asthma has a higher prevalence in boys before puberty and a higher prevalence in women in adulthood. Generally, around puberty girls will have a higher incidence of asthma31 and this can be supported by wheeze being more common in our females.
Our results relied on self-reported information and could have been affected by information bias. However, the ISAAC phase 3 questionnaires are standardised and have been broadly used internationally. A further possible limitation is that urban–rural status was defined on the basis of current residency. We did not know if the urban respondents had previous rural dwelling experience, as migration of rural persons into cities is common in our country. As child's early life exposures are of great importance in asthma development, this might affect our results. Most respondents were residing in urban areas and in North/Western Macedonia where the asthma prevalence was higher. However, it is likely that the urban–rural differences in asthma would be more prominent without rural migrants in urban areas. There were no data on the farming background of the rural respondents; however, most of them would be expected to be farming according to the rural lifestyle in our country. Another limitation was that the question about pet ownership included both the close contact with pets at home and also pets outdoors. Finally, related to diet as a mediating factor in the urban–rural associations with current wheeze and asthma, there was a lack of data on consumption of specific types of fruit, vegetables, fish, and milk and the way of their storage and processing before consumption.
In summary, a lower prevalence of asthma was found in rural compared to urban dwelling Macedonian adolescents although the association was not statistically significant after adjustment for other factors, suggesting that the effect is mediated by at least one of these other factors, explaining the association. The association between urban–rural status and current wheeze or asthma was mediated by the region of the country, with diet likely as a part of the reason for this mediating effect. Having a dog resulted in a strengthening of the association between urban–rural status and current wheeze. This supports the hypothesis that the environment can explain the differences in asthma between urban and rural areas. Further studies are needed to investigate in greater detail the environmental and lifestyle factors, especially diet, in different regions of the country and their impact on urban–rural differences in asthma prevalence. Irrespective of where adolescents live, under-diagnosis of asthma should be considered as an important problem in the Republic of Macedonia and managed adequately.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans and animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflict of interest to declare.
We are grateful to the young adolescents who participated in the study, and the assistance by the school stuff is much appreciated. The authors also thank all the colleagues listed in Appendix A for the participation in data collection and entry in data set related to the study that has given rise to the article and to GlaxoSmithKline Macedonia for the financial support of the study.
Skopje: E Vlaski (principal investigator), K Stavric, L Seckova, M Kimovska, R Isjanovska, A Sazdovski, I Kirovski, A Iseni; Ohrid: S Arsenova, G Sotirovski, O Zekir, K Dimitrova; Tetovo: S Asani, T Jakupi, V Tomic, K Ramadani; Veles: B Cuskova, R Bosilkova, A Varsamis, C Jordanova Kostova; Berovo: V Markovska, M Radinska; Delcevo and Makedonska Kamenica: V Arsova; Pehcevo: N Simovska.