INTRODUCTION
Knowledge on etiology, genetics, clinical aspects and management have deeply improved the outcome of patients affected of Primary Immunodeficiencies (PID) in last years. This article reviews the main modalities of management currently used in the field, and it is the result of an activity of the Immunology Working Group, Spanish Society of Pediatric Clinical Immunology and Allergy (SEICAP).
IMMUNOGLOBULIN1,2
In 1809, Behring and Kitasato showed the utility of immune sera for providing protection against infections; decades later, their utility in the prevention of infections such as measles, tetanus, diphtheria and hepatitis A was demonstrated. In 1952, the first treatment with human serum immunoglobulin (Ig) was performed in a case of Bruton-type Congenital Agammaglobulinemia; since then, and up to 1981, the use of intramuscular Ig became standard treatment, being replaced subsequently by intravenous Ig.
Igs are obtained by alcoholic fractionation of a pool of human sera derived from Cohn fraction II; this procedure eliminates other proteins and lives viruses (hepatitis B virus, HIV, HCV), giving rise to a sterile product for intramuscular or subcutaneous injection. The preparations obtained the WHO guidelines contain thiomersal as the preservative, glycerol as the stabiliser and have a pH of 6.8, yielding a product with 95 % IgG, at a concentration of 16.5 % (165 mg/ml), containing all the IgG subclasses and multiple IgG allotypes (Gm and Km), with traces of IgM, IgA and other serum proteins. This preparation contains a broad spectrum of antibodies to viruses and bacteria.
Intramuscular immunoglobulin
In vitro, it has been demonstrated that IMIg produces aggregates of IgG with high molecular weights that activate the complement system and are responsible for the systemic reactions that are sometimes observed. The incidence is higher if the person to whom it is administered has previously received IgG or if it is accidentally administered intravenously these preparations are therefore contraindicated by this route.
Today, in our setting, IMIg is practically never used for the treatment of the primary immunodeficiencies and its use is limited to the prevention of several infections (HAV, HBV, measles, tetanus, rabies and Central European tick-borne encephalitis).
Intravenous immunoglobulins
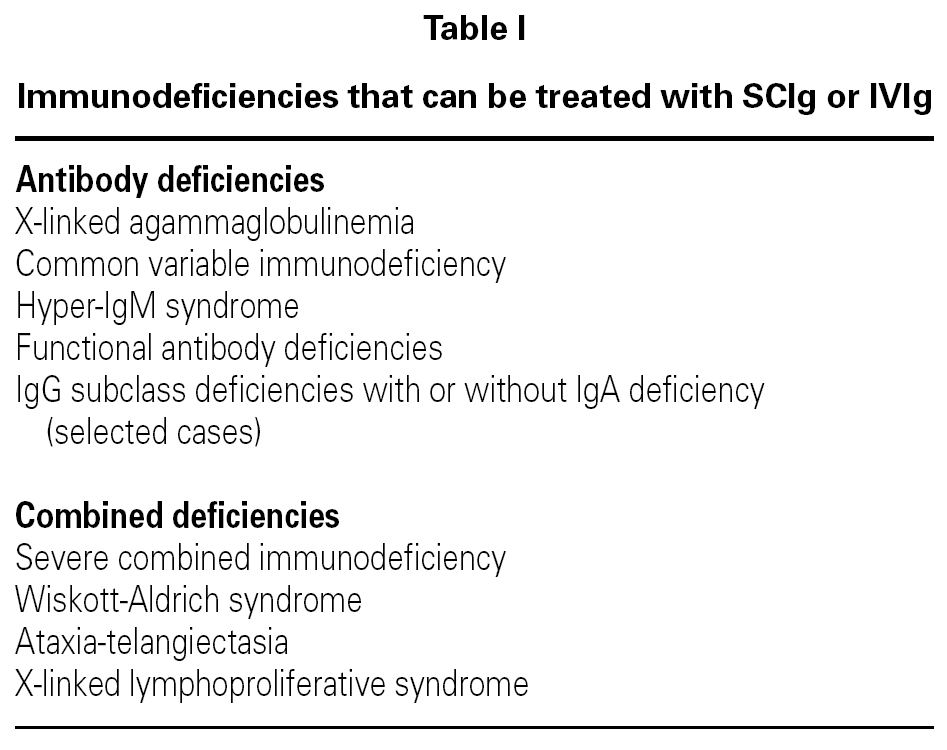
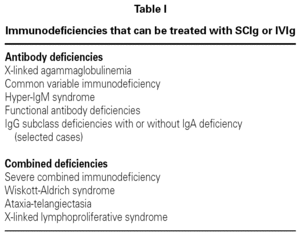
Treatment with IVIg is the most widely used for the treatment of primary and secondary immunodeficiencies (table I). It has significant advantages, including the easy administration of large doses, rapid onset of action, absence of proteolysis of the product and its administration is painless.
All the available preparations approved by the FDA and EMEA have a half-life of 18 to 25 days, contain all the IgG subclasses, have minimal anti-complement activity, have a broad spectrum of antibodies and are free of hepatitis B and C viruses and HIV. HCV infection did occur in 88 of 137 patients who received an IVIg product in October 19943, 58 % of whom had a PID. This obliged the manufacturers to employ other methods to inactivate HCV and other viruses.
The recommended dose to avoid infections4 or hospitalisations and to improve lung function is 400 to 600 mg/kg, aiming to maintain an IgG level of over 500 mg/ml or 350 mg/ml over the baseline level. In patients with chronic lung disease, chronic diarrhoea or growth failure, the doses must be higher until the clinical situation is controlled5.
Administration requires a venous access and an infusion over several hours (between 2 and 4). The infusion must be started at a rate of 0.5 mg/kg/minute or 0.001 ml/kg/minute), with the rate of administration being doubled every half-hour if no adverse reaction occurs, up to a maximum of 2-3 mg/kg/minute or 0.004-0.006 ml/kg/minute.
The adverse effects of the IV infusion of gammaglobulin can be due to an excessively rapid administration to patients who receive it for the first time, patients with infection at the time of the infusion, or if more than four to six weeks have passed since the previous dose was administered.
The adverse effects reported include: headache, nausea, vomiting, rigors, joint pain and/or abdominal pain. These occur in 5-15 % of patients receiving IVIg and can be minimised by pre-treatment with oral paracetamol or antihistamines. Allergic reactions such as urticaria and anaphylaxis rarely occur. Severe reactions require treatment with adrenaline, corticosteroids and antihistamines.
When a severe reaction has occurred, pre-treatment must be given with paracetamol, antihistamines and an IV infusion of hydrocortisone 6 mg/kg (with a maximum dose of 100 mg) administered one hour before the infusion of the IVIg, with the dose being repeated at four hours if the infusion of Ig has not finished.
Although rare, some late reactions have been reported with high doses of IVIg used as immune modulators. These include aseptic meningitis6, cerebral thrombosis7, disseminated intravascular coagulation, renal and respiratory failure8 and haemolytic anaemia9.
IVIg is contraindicated in patients with a history of anaphylactic reactions to IVIg or other blood products. It must be administered with great caution in cases of patients with deficiencies of subclasses of IgG with IgA deficiency and/or anti-IgA antibodies, as these patients are at risk of severe reactions3,10.
Subcutaneous immunoglobulins11
SCIg, used for years in the north of Europe as prophylaxis in humoral immunodeficiencies, is an alternative to IVIg for the treatment of primary immunodeficiencies. As the technique for inserting a small perfusion needle subcutaneously is simple and there are no reports of adverse effects, the medication can be self-administered into the abdominal wall or thighs. The injections are well tolerated. The local reactions are minimal and include erythema and/or pain; systemic reactions are rare.
The monthly dose used is the same but is divided over four weeks. Initially, the concentration of the SCIg is 16 %, to be infused at a rate of 0.05 to 0.2 ml/ kg/hour or 1-3 ml/hour, with the aid of a small, battery-powered perfusion pump.
The standard doses used are of 100 to 150 mg/ kg/ week, corresponding to a dose of 45-60 ml of a 16 % solution for a 70-kg patient. Injection sites are the four abdominal quadrants and the lateral region of the thighs or forearms; the infusion of more than 15 to 25 ml of solution is not recommended at any site and many patients therefore give themselves infusions of 20 ml per site at intervals of one hour and find this perfectly satisfactory. The change of treatment from an IVIg to SCIg must be performed with an interval of one week between the final dose of IVIg and the first dose of SCIg. Patients diagnosed for the first time and who have not previously been treated with Ig of any type can start the replacement treatment directly with SCIg with daily doses of 100 mg/kg for five consecutive days, followed by weekly maintenance doses.
In children and infants, the subcutaneous infusion of 2-3 ml per site can be performed directly, every 5 minutes, without a perfusion pump. Depending on the size of the child, one or two doses per week can be sufficient.
Comparative studies of the efficacy and safety of IVIg and SCIg for replacement therapy found no significant differences with respect to the number of infections or adverse reactions, and recent studies have shown that SCIg administered at home is associated with better quality of life than IVIg administered in the hospital; it also gives the patients and their families greater independence and greater control over aspects of treatment and of their daily life. Learning how to administer the SCIg is easy and represents an alternative to the IVIg.
This route has been used by a number of authors in patients who had previously suffered anaphylactic reactions with IM or IVIg, when venous access is difficult or when an episode of aseptic meningitis has occurred after the use of IVIg.
The Scandinavian experience has demonstrated that an SC dose of 100 mg/kg/week of Ig has fewer adverse effects (30 from 3,232 doses, 0.9 %) than IM administration (442/1,893, 23 %) or IVIg (179/387, 46 %) (10 and 11). The serum levels of IgG achieved were similar to those reached with IVIg. A number of products have been marketed specifically for the subcutaneous route and to avoid the Ig preparations with thiomersal in order to prevent possible mercury intoxication; some authors recommend the use of 10 % IVIg, stating that this is well tolerated by the subcutaneous route.
STEM CELL TRANSPLANTATION
That is a procedure aimed at restoring the number or function of haematopoietic cells (Primary Immunodeficiencies and other congenital haemopathies, or after bone marrow aplasia resulting from the treatment of acquired neoplastic or autoimmune processes), of the cells of the macrophage-monocyte system (haemophagocytic syndromes) or to provide a source for the replacement of enzymes (congenital metabolic diseases)12-16.
It is used in those PIDs (of T lymphocytes and phagocytes) which would otherwise almost certainly be fatal, with high morbidity or with a negative impact on quality of life. Severe combined immunodeficiency is a true diagnostic and therapeutic emergency in paediatrics17.
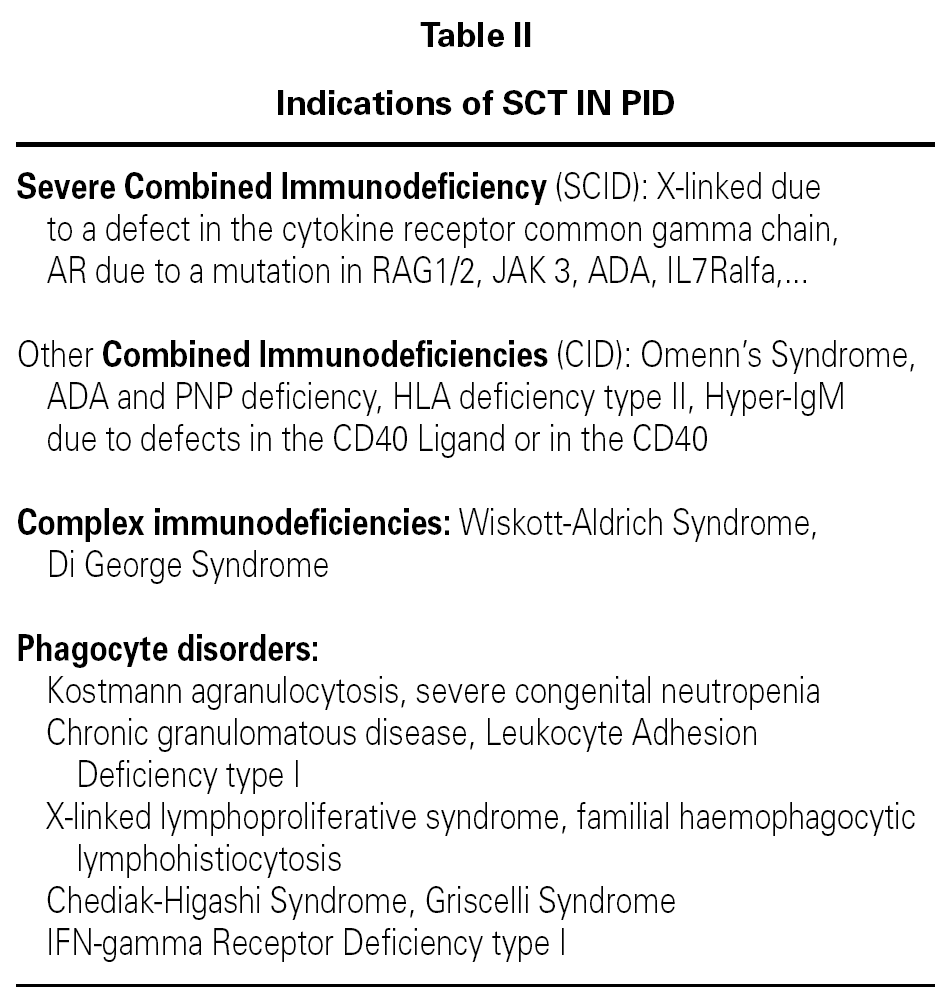
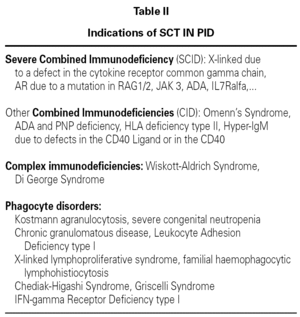
The SCT is indicated in T lymphocyte PID (combined or isolated) and phagocyte defects (table II)12,15,18.
Donor selection
Syngenic identical related (homozygotic twin) or allogenic transplant. Matched unrelated allogenic transplant. Partially matched transplant: haploidentical related donor19-22.
For SCT in PID, unmatched unrelated donors are not used due to the high risk of severe GVHD caused by this situation. The choice is based on availability, condition of the patient and urgency, as well as on the underlying disease.
Sources of stem cells
1.Bone marrow.
2.Cord blood: this is a rich source and produces hundreds of times more CD34 + CD38 stem cells in culture than bone marrow or peripheral blood, and these cells have different immunological properties, causing less GVHD. Since 1988, more than 4000 SCT have been performed using cord blood and there are more than 150,000 cords stored in the different bone marrow banks throughout the world23.
3.Peripheral blood (mobilisation of CD34 + stem cells to the periphery using G-CSF, with leukapheresis on day 5-7)24.
Thymus transplantation (foetal or postnatal) is not exactly a stem cell transplantation. It is indicated in the Di George Syndrome25.
Pretransplant preparation
Laminar flow room. Low germ diet. Central venous line. Ensure nutrition (if necessary, use Total Parenteral Nutrition or Continuous Enteral Nutrition).
Prophylaxis: Non-absorbable oral antibiotics (colimycin, neomycin, vancomycin). Antifungal agents (fluconazole, amphotericin). Co-trimoxazole. Aciclovir-ganciclovir. Irradiation of blood products, if required.
Graft-versus-host-disease (GVHD)26,27
This is due to the recognition of the host tissues by the donor T lymphocytes ("reverse rejection"). If an HLA Class II mismatch exists, the disorder is usually very severe or even fatal. The minor histocompatibility antigens give rise to a greater or lesser degree of GVHD despite the matching (even if "complete") in the majority of patients transplanted for PID. It is more common and/or intense with unrelated donors due to the possible higher degree of mismatch. It may be:
Acute: in the first three months post-transplant. It affects the skin (rash), bowel (diarrhoea) and liver (dysfunction). It has four grades of severity27.
Chronic: from 100 days post-transplant. This occurs in many patients who suffer moderate or severe acute GVHD. Predominance of "autoimmune" manifestations (cytopenias).
Prevention: Selection of the best-matched donor possible. Immunossupression prior to SCT (calcineurin inhibitors: cyclosporine or mycophenolate). Depletion of mature donor T lymphocytes: reduces the GVHD but increases rejection and infections. Produces "mixed chimerism" (cells of different origin, donor T lymphocytes and host B and myeloid cells).
Methods of depletion: destruction (monoclonal antibodies) or extraction of the T lymphocytes (separation by rosettes), extraction of CD34 + stem cells (by magnetic microbeads; positive selection).
Treatment of GVHD: Increase the immunossupression (corticosteroids and calcineurin inhibitors,...), which also further increases the risk of infection.
Pretransplantation conditioning14,28-30
Indicated when there is residual T-lymphocyte immunity (combined immunodeficiencies, phagocyte disease) to prevent graft rejection. The protocols used in SCT for PID are still not well standardised, and must be adapted in each centre to the characteristics of the individual patient and to the advances that are taking place in this field; effective "reduced intensity" regimens now exist with a lower toxicity than those used previously. For this reason, it is important that SCT is performed in centres with extensive experience in these types of patients, with adherence to the current recommendations of the experts in the field. The reader is referred to the literature references3,23.
Graft
A Chimera31 is a cell line that is foreign to the host and that comes from the donor. Its existence and grade can be determined by various methods (the most simple being karyotyping if the donor and host are of different sexes, and up to PCR microsatellite amplification)1,32.
The degree of chimerism that is curative varies depending on the genetic disease for which SCT is performed. In the majority of the PIDs, as there is no T-cell function, ablative conditioning is not performed (or is of low intensity) and the B lymphocytes and myeloid series principally of host origin persist in coexistence with the chimera of donor T lymphocytes; for this reason, the persistence of the host B lymphocytes in many cases of SCID means that antibody immunity does not recover. In other cases, the thymic microenvironment in children with SCID is able to induce the differentiation and functional maturation of the donor stem cells into T lymphocytes that can co-operate with apparently normal B lymphocytes from the host in order to form antibodies33.
Graft kinetics33,34
The T lymphocyte chimera and its onset of function can develop between the second and fourth week after the transplant in matched cases, and after the second month with a maximum between the third and fourth month in haploidentical transplants, with functional normalisation in the fourth to seventh month.
In many cases there is a non-chimeric persistence of the host B lymphocytes, though the normalisation of their function (antibody formation) often takes more than two years, if it happens at all.
Prognostic factors20,35-38
Age: early transplantation (three to six months of life in SCID, under five years in Wiskott-Aldrich syndrome) is substantially better. Intrauterine SCT has been performed but the results do not appear to be better than those performed in the newborn, and the procedure is more complicated39.
Degree of matching: DR (maximum importance), minor antigens.
Related-unrelated
Active infections (CMV, RSV...) prior to the SCT
Possibly, in the future, the performance of related haploidentical SCT, performed early because of availability, with the better prognosis after improvements in the techniques of T lymphocyte depletion, may represent a highly effective therapeutic option.
Post-transplantation problems
1.Secondary immunodeficiency. Post-transplantation infections27,40:
Immediate post-transplantation phase (up to 30 days). The neutropenia and breakdown of the mucocutaneous barrier (catheters, mucositis) favour bacterial infections (gram positive and negative).
Intermediate phase (30 to 100 days), immunosuppression and GVHD. Fungal infections may develop due to inhalation of spores (Aspergillus) or via catheters (Candida,...). The risk of Pneumocystis jerovici and viral infections also persists, these may be: nosocomial (RSV, influenza and parainfluenzae), due to reactivation of latent viruses (CMV, adenovirus) or both (herpesvirus, post-SCT lymphoproliferative disease (EBV).
Late phase (after 100 days). Initial (or persistent) antibody deficiencies also give rise to more infections (capsulated organisms, varicella-zoster).
Prevention and treatment: Isolation (laminar flow room) and absolute asepsis. Aciclovir (prophylaxis for herpes virus). Systematic detection of viral reactivation (PCR on the blood) and prevention/early treatment (ganciclovir or forcarnet for CMV, rituximab anti-CD20 in lymphoproliferative disease due to EBV). Fluconazole or itraconazole (prophylaxis), or liposomal amphotericin in Candidal infections. Cotrimoxazole (prophylaxis and treatment of Pneumocystis carinii).
Treatment of bacterial infections (St. aureus: vancomycin; Pseudomonas aeruginosa cefepime/ amikacin; gram negative: ciprofloxacin,...). IV gammaglobulin. Donor granulocytes pre-treated with G-CSF.
A possible consideration in the future may be antiviral vaccinations to the donors prior to transplant.
2.Hepatic veno-occlusive disease: Pre- and post-transplant anticoagulant prophylaxis with heparin.
3.Late endocrine effects41: On growth hormone, thyroid function, sex hormones and reproductive function, and on the pancreas.
4.Late non-endocrine effects42-44: Cardiac, pulmonary, renal, neuropsychological, neurological, ocular, dental, salivary and bone disorders can occur, as well as ototoxicity, tumours, altered quality of life and late mortality.
GENE THERAPY
Gene therapy, as it is currently employed, is the therapeutic strategy based on changing a defective gene or adding a normal gene to a patient in order to correct functional deficiencies in certain cells necessary for various physiological functions45,46. This therapeutic technique has been tested on a number of occasions in recent years but, to date, the results have not been encouraging. Advances in the methodology used would augur better therapeutic perspectives.
All the cells of our body contain the complete human genome (and, hence, the defective gene in patients with genetic defects). However, not all cells are essential for performing certain functions. Thus, in the case of haematopoietic cell diseases, such as the primary immunodeficiencies (PID)47, the genetic defects in these cells must be corrected. This has made the PIDs the first diseases in which gene therapy has been used48,49 and the first in which the efficacy of this treatment has been demonstrated50,51.
Methods
In order to introduce a healthy gene into the cells that we wish to "correct", vehicles or vectors, usually in the form of viruses, particularly retroviruses52,53, are used after their manipulation to prevent their replication within the cells, though maintaining their "infective" capacity. These vectors enable the healthy gene to be incorporated into the cell genome and thus achieve the stable expression of the gene.
The transfection is performed "ex-vivo" by the incubation of the cells in culture with the viral vector containing the desired gene and their subsequent reintroduction into the patient (around 300 million corrected cells) by a simple transfusion. In some cases, the direct administration by systemic injection or injection into the affected organ is being tested54.
The insertion of the healthy gene into the cell genome "close" to other genes such as those responsible for tumours (proto-oncogenes) gave rise to an uncontrolled proliferation of these cells and the appearance of leukaemias55 in some of the cases of severe combined immunodeficiency (SCID) treated in the Necker Hospital in Paris.
These problems have not been observed in the protocols performed by other groups but, to avoid them, a number of research studies are under way on how to control and regulate the insertion of the gene, what are the most suitable types of vector, what quantities must be used, etc., which will help to perfect the techniques and obtain better results, avoiding the adverse effects56.
Other undesirable effects can include the inactivation of essential tumour suppressor genes. However, these effects would be beneficial in the case of gene therapy for tumour processes57, blocking activated oncogenes, replacing inactivated tumour suppressor genes or adding cell apoptosis-facilitating genes, etc.
Other methods used:
1.The use of other viruses such as adenoviruses (58), or adeno-associated viruses (AAV), smaller viruses not associated with disease, and herpes viruses. However, it is known that the majority of people have antibodies to adenoviruses, reducing the efficacy of the technique. Furthermore, a case of death has been reported in the USA, possibly due to excessive doses of the adenovirus giving rise to an uncontrolled immune reaction.
2.Physical methods: direct microinjection, electroporation, etc.
3.Chemical methods: using chemically-modified viral vectors and even by the synthesis of artificial viruses, or using synthetic substances such as liposomes, polymers, etc.
RESULTS AND DISCUSSION
There are many candidate diseases for gene therapy and a number are in clinical trials59. The majority are haematological diseases, such as thalassaemia and haemophilia60, metabolic diseases61, etc. However, there is also great hope for the treatment of certain tumours and for the treatment of AIDS by the suppression of viral genes62, for example.
Why are severe PID ideal candidates for gene therapy? PIDs are diseases caused by molecular defects, each due to a single defective gene (monogenic diseases) responsible for the functional abnormality of the haematopoietic cells. These cells are the precursors of all the cells of the immune response and, as mature cells, they emigrate to the whole lymphoid system and are able to perform the functions specific to each one of them. In addition, haematopoietic cells are easy to obtain and culture, and maintain their capacity of replication, ensuring a high level of efficacy.
The first treatments performed in humans in the 1990s48, in SCID due to ADA deficiency, were not very positive: the synthesis of this enzyme decreased very rapidly and the immunological defect was not corrected. More recently, after many experimental studies, a protocol has been initiated for the treatment of severe combined immunodeficiency (SCID), specifically in the form with an X-linked inheritance (a defect in the cytokine receptor common gamma chain) for patients who do not have a matched donor for stem cell transplant (SCT)37. A number of treatments have now been performed in various European centres (Paris, Milan and London) with slightly varying protocols. Treatment has also been performed in cases of ADA deficiency.
The problems encountered by the Paris group, the appearance of three cases of ALL in a total of 11 children treated, were caused by the capacity of the vector to activate proto-oncogenes or inactivate tumour suppressor genes after their insertion into the genome. This led to the interruption of the treatments, though they have now been restarted after intense research into the points of insertion of these vectors, the amount of vector used, etc.
In 2006, the results of the treatments with gene therapy in two adults suffering X-linked chronic granulomatous disease (X-CGD) were published by the Institute of Biomedical Research in Frankfurt63. The initial results were very good but the neutrophils did not maintain their bactericidal activity indefinitely.
The results, despite the consequences described above, are very encouraging. Firstly, it has been shown that gene therapy works, that it is able to provide a permanent reconstruction of the function of lymphoid cells (presence of the full cytokine receptor), that it has "cured" the defect, and that some patients are still alive after four or five years with no problems. Despite the difficulties that have occurred, the reconstitution of normality has been demonstrated in these cases (with normalised lymphocyte function) and many studies are being performed64,65 on the different regimens and different indications in very diverse diseases, which will come to fruition in the near future.
In conclusion, therapy of PID has changed a lot in the last 15-20 years and a near normal life is obtained in most patients, if diagnosis is made correctly and therapy measures are precociously established; in addition, we can expect big advances in next years which will probably improve even more the prognosis of PID patients.
Correspondence:
Dr. Juan M. García Martínez
Pediatric Allergy and Immunology Unit
Hospital de Cruces
Pza. de Cruces, s/n
48903 Baracaldo. Vizcaya. Spain
E-mail: juanmiguel.garciamartinez@osakidetza.net