Asthma symptoms can be triggered by a variety of factors commonly referred to as “triggers”. Some of these factors can also induce severe asthma exacerbations. Thus, it can be assumed that actions taken against such triggers may prevent the progression of the disease. However, limited data exist on the clinical importance of these triggers in patients with chronic obstructive pulmonary disease (COPD).
ObjectiveTo compare the effect of triggers on symptoms and actions taken against certain modifiable triggers in patients with asthma and COPD.
MethodsThe study was conducted in a university hospital between June 2009 and June 2010. Patients with asthma and COPD were asked to complete a questionnaire in which both the factors triggering symptoms and the actions taken against several triggers were assessed.
ResultsThree hundred consecutive adult patients (150 asthma, 150 COPD) were enrolled to the study. The frequency of triggering factors was similar in both groups. Vaccination rates for influenza and pneumococcus were significantly higher in patients with COPD. However, such anti-allergic approaches as the use of strategies to decrease dust exposure, the use of anti-mite bed sheets, and the removal of pets from the home were more commonly employed by asthmatic patients.
ConclusionThis study revealed that certain triggers affected COPD and asthma patients to the same degree. Therefore, triggers and strategies for controlling modifiable triggers should be more concentrated on during education in both groups. However, the preventive effect of these strategies on disease progression, particularly in patients with COPD, needs clarification.
Chronic airway diseases such as asthma and chronic obstructive pulmonary disease (COPD) are among the most common respiratory diseases of our age, affecting an estimated 300 million people plus worldwide.1,2 While the treatment plan for both of these diseases is mainly based on symptom relief and controlling the underlying inflammatory process, prevention of the progression of the disease is also another important objective.1,2 To date, the number and frequency of attacks, and in particular severe attacks, have been shown to be associated with the progression of both diseases, particularly in the case of asthma.3–5 Therefore, strategies to prevent severe attacks from occurring may lead to a better outcome in both diseases.
Asthma symptoms can be triggered by a variety of factors, commonly referred to as “triggers”, which consist of both specific factors, such as allergens, and non-specific factors, such as viral infections, pollutants, etc.6 While allergens cause symptoms in sensitised patients upon exposure, non-specific factors may trigger any asthmatic patient regardless of their underlying atopic status, even though some differences do exist on an individual basis.1,3 Some of these factors are also capable of inducing severe asthma exacerbations.7,8 Thus, it can be assumed that actions taken against such triggers may prevent the progression of the disease. The relationship between triggers and asthma symptoms has been well defined in the literature.6,9–11 Although the role of bacterial infections on symptom exacerbations is well known, the importance of other triggering factors is not so clear for patients with COPD.
It is also of interest what kind of actions are taken against certain triggers in these patients, as preventive measurement is one part of asthma management.1 In our previous work, we showed poor compliance of asthma patients in protecting themselves against certain triggers.12 To our knowledge, the behavioural attitude of COPD patients to take preventive measures against certain common triggers has yet to be studied. Therefore, in this study, we studied non-specific triggering factors in patients with asthma and COPD to see whether the effect of triggers and the actions taken by the patients against certain triggers were different.
Material and methodsPatient selection and the study designThe study was conducted in the Allergy and Pulmonology clinics of a university hospital between June 2009 and June 2010. A total of 150 adult patients with asthma and 150 adult patients with COPD were consecutively enrolled into this cross-sectional study. Asthma diagnosis was based on a prior history of recurrent wheezing, shortness of breath, cough, and demonstration of objective signs of reversible airway obstruction by means of at least >12% and 200ml increase in FEV1 after 15min with an inhalation of 200μg salbutamol.1 A diagnosis of COPD was defined according to the GOLD standards.2 Inclusion criteria included: (1) a history of dyspnoea, chronic cough or sputum production, and/or a history of exposure to risk factors for the disease, especially cigarette smoking; and (2) a FEV1/FVC ratio of less than 0.70.2 Based on history, physical findings and laboratory investigations, the cases in which asthma and COPD distinction cannot be made were not included in the study.
Demographics and disease characteristics were recorded at the start of the study. Current asthma activity was assessed via the “Asthma Control Test” (ACT), which is validated in Turkish.13,14 In the test, a score of 25 points shows fully controlled asthma, 20–24 points corresponds to well-controlled asthma, and a score of less than 20 points shows uncontrolled asthma. For COPD, dyspnoea was evaluated according to the Medical Research Council (MRC) scale. The MRC 5-grade scale is a five-point scale based on degrees of various physical activities that precipitate dyspnoea15 with increasing severity from scores “0” to “5”.
The skin prick test results of the asthmatic patients were collected from hospital records. The atopic status of the COPD patients was not known.
The patients were asked to complete a questionnaire in which the first section assessed factors triggering symptoms. This questionnaire was developed from a questionnaire the authors had used in their previous study.12 The questionnaire consisted of related topics with either yes/no questions or multiple choice questions. The triggers chosen for inclusion in this study were taken from the most common and well-defined factors previously recorded and, as such, are the ones generally placed in international guidelines. Each trigger was listed separately and patients were asked to highlight the name(s) of triggers which caused deterioration in their symptoms. Female subjects were also asked to give information about gender specific triggers such as pregnancy and menstrual cycles. The questionnaire also contained questions included to document information concerning actions taken by patients against certain triggers and whether they had made any changes to their lifestyle following their asthma/COPD diagnosis. The study was approved by the local ethics committee of Ankara University. All patients gave written informed consent to be included to the study.
StatisticsThe statistical analyses were performed by a computer software package (SPSS version 11.0, Chicago, IL, USA). Descriptive statistics were expressed as mean±SEM and n (%). Categorical data were tested by Chi-square test. One-way ANOVA was used for the comparison of continuous variables in different asthma controls and MRC scores. A p value less than 0.05 was considered statistically significant.
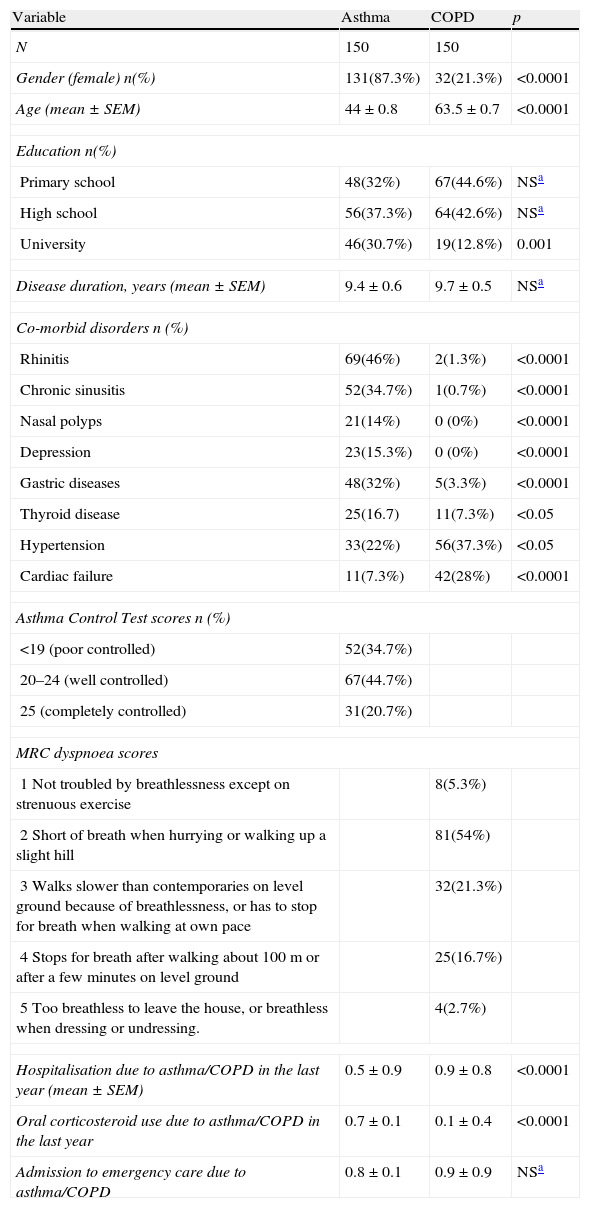
ResultsStudy groupThe study consisted of 150 asthma and 150 COPD consecutively enrolled patients. The majority of patients in the COPD group were male, and the group showed more frequent cardiac co-morbidities in comparison to patients with asthma. In contrast, asthma patients included in the study were predominantly female, had a higher educational level, and were observed to have more rhinitis, chronic sinusitis, nasal polyps, thyroid diseases, depression, and gastric diseases as co-morbid diseases (Table 1). Patients with COPD had been hospitalised more frequently in the past year and had been admitted to emergency care more times than the asthma patients in the study (Table 1). One-fifth (n=31, 20.7%) of the asthmatic patients had completely controlled asthma. The majority of the patients with COPD had a MRC score of 2 (n=81, 54.7%) (Table 1).
Demographics and disease characteristics of the study groups.
| Variable | Asthma | COPD | p |
| N | 150 | 150 | |
| Gender (female) n(%) | 131(87.3%) | 32(21.3%) | <0.0001 |
| Age (mean±SEM) | 44±0.8 | 63.5±0.7 | <0.0001 |
| Education n(%) | |||
| Primary school | 48(32%) | 67(44.6%) | NSa |
| High school | 56(37.3%) | 64(42.6%) | NSa |
| University | 46(30.7%) | 19(12.8%) | 0.001 |
| Disease duration, years (mean±SEM) | 9.4±0.6 | 9.7±0.5 | NSa |
| Co-morbid disorders n (%) | |||
| Rhinitis | 69(46%) | 2(1.3%) | <0.0001 |
| Chronic sinusitis | 52(34.7%) | 1(0.7%) | <0.0001 |
| Nasal polyps | 21(14%) | 0 (0%) | <0.0001 |
| Depression | 23(15.3%) | 0 (0%) | <0.0001 |
| Gastric diseases | 48(32%) | 5(3.3%) | <0.0001 |
| Thyroid disease | 25(16.7) | 11(7.3%) | <0.05 |
| Hypertension | 33(22%) | 56(37.3%) | <0.05 |
| Cardiac failure | 11(7.3%) | 42(28%) | <0.0001 |
| Asthma Control Test scores n (%) | |||
| <19 (poor controlled) | 52(34.7%) | ||
| 20–24 (well controlled) | 67(44.7%) | ||
| 25 (completely controlled) | 31(20.7%) | ||
| MRC dyspnoea scores | |||
| 1 Not troubled by breathlessness except on strenuous exercise | 8(5.3%) | ||
| 2 Short of breath when hurrying or walking up a slight hill | 81(54%) | ||
| 3 Walks slower than contemporaries on level ground because of breathlessness, or has to stop for breath when walking at own pace | 32(21.3%) | ||
| 4 Stops for breath after walking about 100m or after a few minutes on level ground | 25(16.7%) | ||
| 5 Too breathless to leave the house, or breathless when dressing or undressing. | 4(2.7%) | ||
| Hospitalisation due to asthma/COPD in the last year (mean±SEM) | 0.5±0.9 | 0.9±0.8 | <0.0001 |
| Oral corticosteroid use due to asthma/COPD in the last year | 0.7±0.1 | 0.1±0.4 | <0.0001 |
| Admission to emergency care due to asthma/COPD | 0.8±0.1 | 0.9±0.9 | NSa |
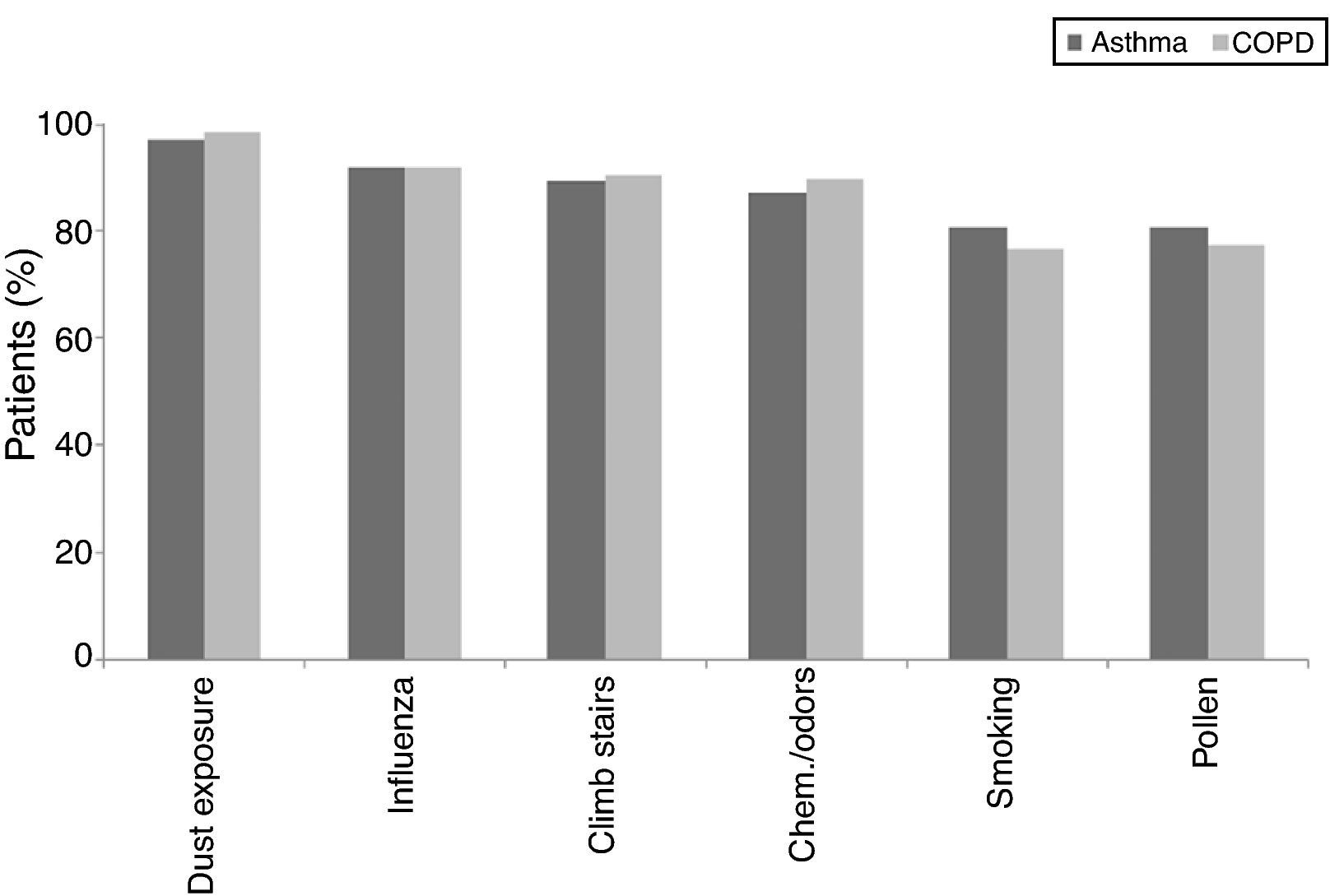
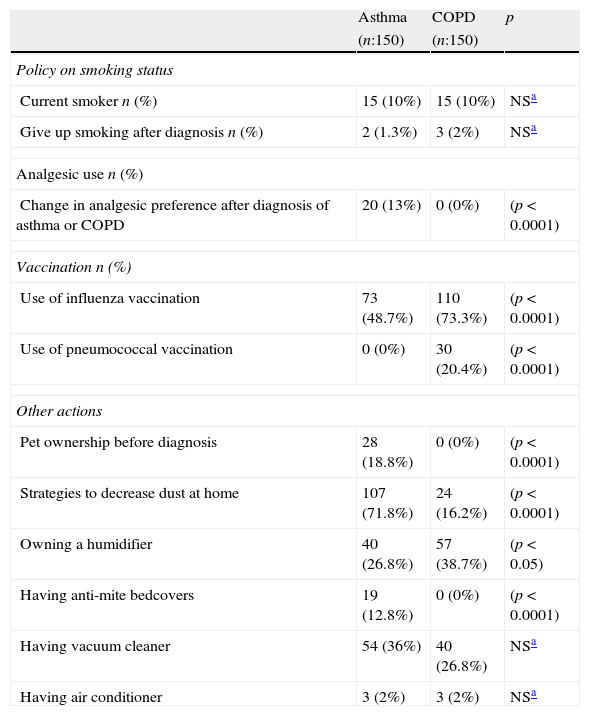
There was no statistical difference in the frequency of the triggering factors in both groups (p>0.05). The most common triggering factor determined in both groups was dust exposure (Fig. 1), followed by upper airway infections and exercise, which were observed at similar frequencies in both diseases. Chemicals/odours, however, were encountered more frequently by asthma patients, and air pollution was more frequent in COPD patients. In female patients, gender specific triggering factors (menstrual cycle and pregnancy) were analysed separately and no statistical difference was found between patients with asthma and COPD.
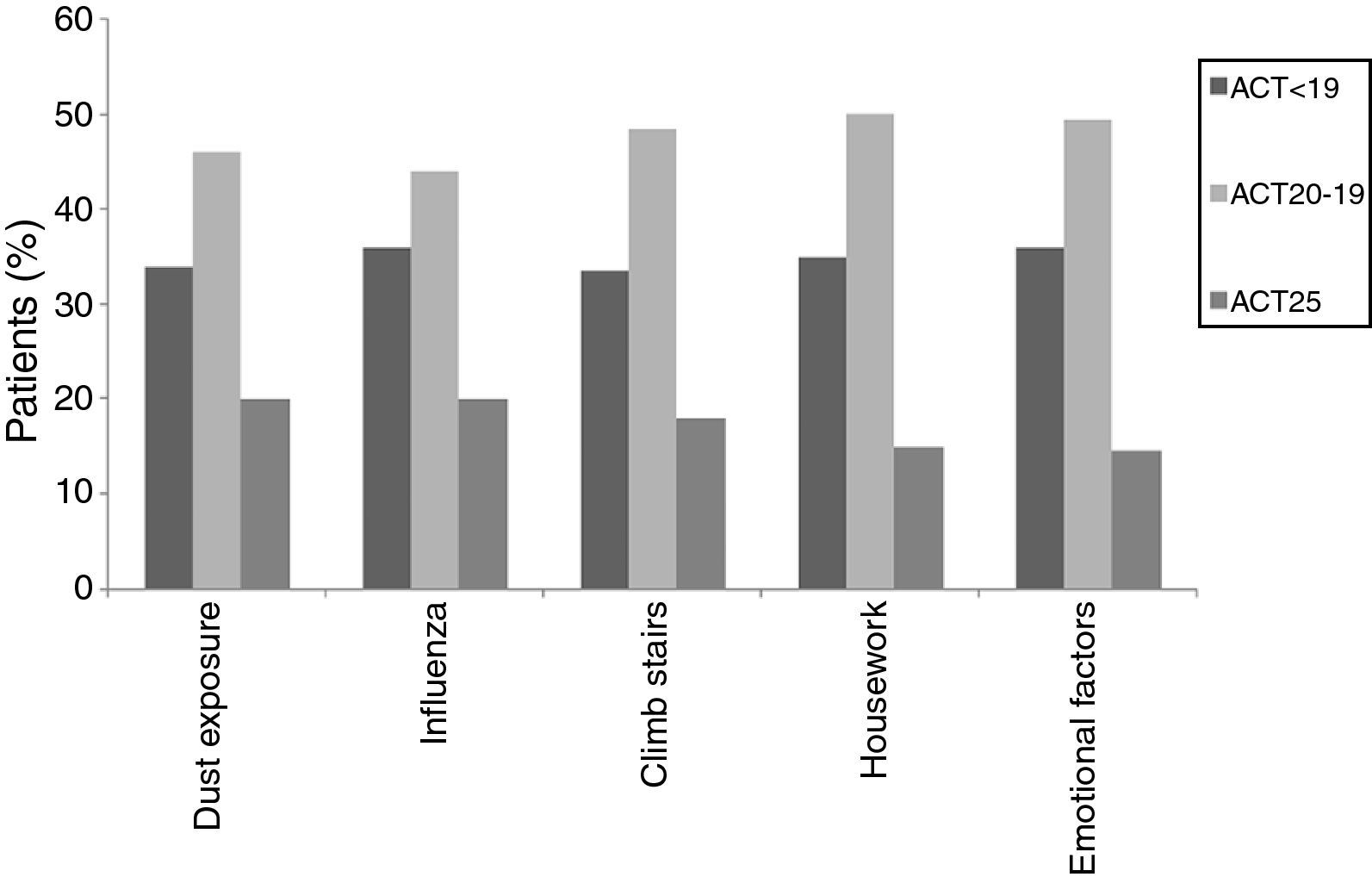
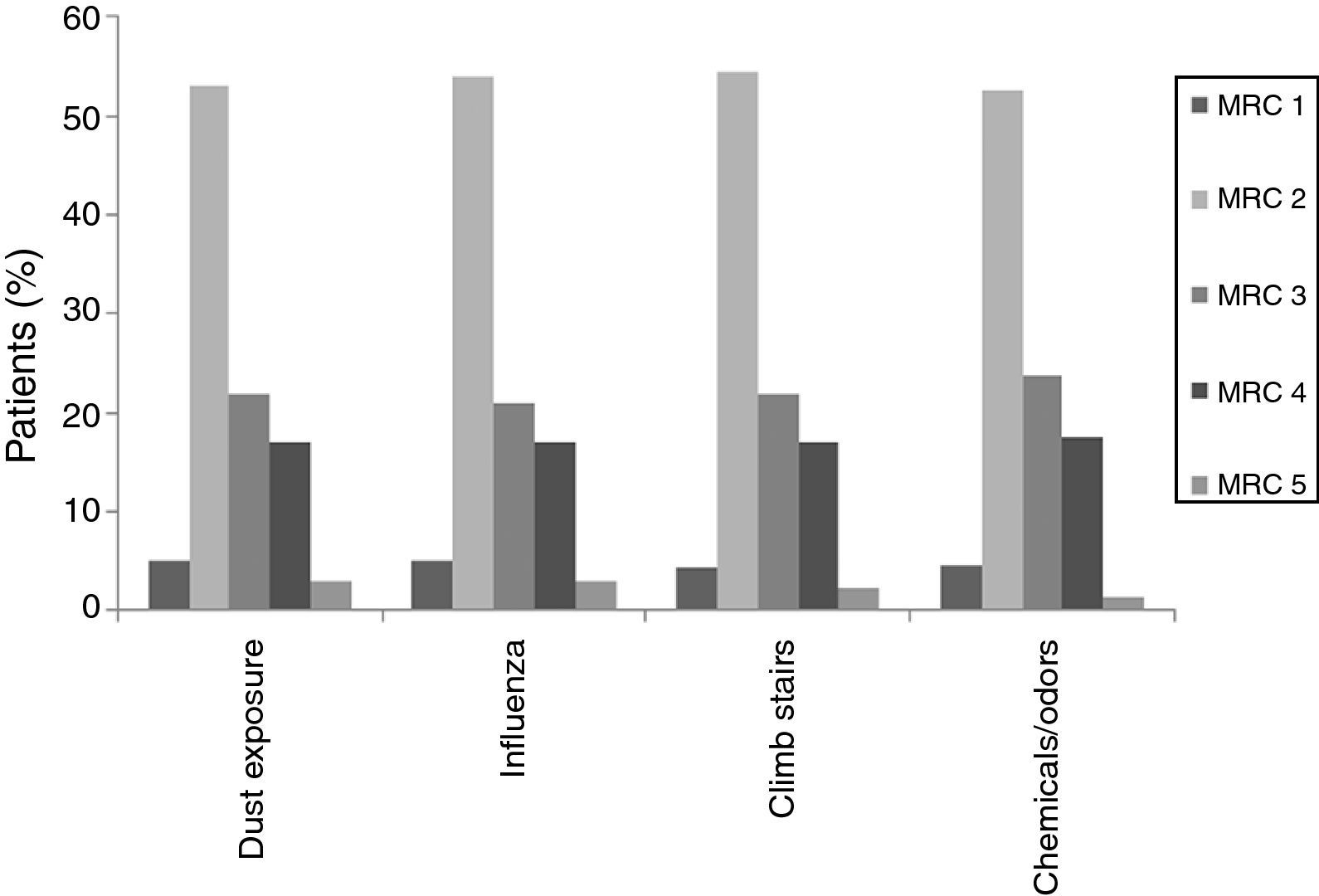
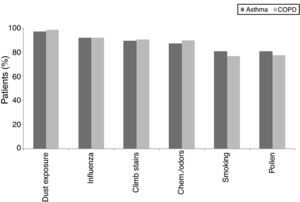
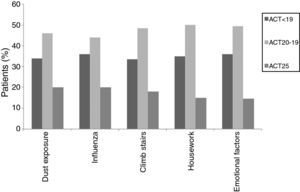
When individual ACT categories were considered for patients with asthma, physical activities such as climbing stairs and doing housework and emotional triggers tended to be more common triggering factors in patients with well-controlled and uncontrolled asthma (p<0.05) (Fig. 2). The triggering factors in the COPD group did not differ according to a variation in the MRC scores (Fig. 3).
Actions against triggersThe actions taken against common triggers and changes made in lifestyles for patients in both groups are shown in Table 2.
Actions against common triggers and life habit changes in each study group.
| Asthma | COPD | p | |
| (n:150) | (n:150) | ||
| Policy on smoking status | |||
| Current smoker n (%) | 15 (10%) | 15 (10%) | NSa |
| Give up smoking after diagnosis n (%) | 2 (1.3%) | 3 (2%) | NSa |
| Analgesic use n (%) | |||
| Change in analgesic preference after diagnosis of asthma or COPD | 20 (13%) | 0 (0%) | (p<0.0001) |
| Vaccination n (%) | |||
| Use of influenza vaccination | 73 (48.7%) | 110 (73.3%) | (p<0.0001) |
| Use of pneumococcal vaccination | 0 (0%) | 30 (20.4%) | (p<0.0001) |
| Other actions | |||
| Pet ownership before diagnosis | 28 (18.8%) | 0 (0%) | (p<0.0001) |
| Strategies to decrease dust at home | 107 (71.8%) | 24 (16.2%) | (p<0.0001) |
| Owning a humidifier | 40 (26.8%) | 57 (38.7%) | (p<0.05) |
| Having anti-mite bedcovers | 19 (12.8%) | 0 (0%) | (p<0.0001) |
| Having vacuum cleaner | 54 (36%) | 40 (26.8%) | NSa |
| Having air conditioner | 3 (2%) | 3 (2%) | NSa |
Fifteen patients (10%) from each group were current smokers. Seven and nine patients with asthma and COPD, respectively, had tried to give up smoking, and two asthmatics and three patients with COPD had succeeded. Passive smoking exposure was reported as a triggering factor at similar rates by all patients (p>0.05).
Actions against analgesic useAlthough none of the patients with COPD were advised to change the analgesics that they used, 20 (13%) patients with asthma were advised to change their analgesics, mostly in favour of NSAIDs, which preferentially inhibit the COX-2 enzyme (p<0.001). Paracetamol was the most preferred analgesic among responders in both the asthma and COPD groups (52% and 65.6%, respectively). Seventeen (11.4%) of the patients had experienced an asthma attack following consumption of an analgesic, whereas none of the patients with COPD had experienced such a relationship.
Actions for vaccinationsNearly half of the asthma patients (48.7%) reported that they had been vaccinated against influenza, and 60% of them routinely had this vaccine each year. However, the vaccination rate for influenza was higher in COPD patients (n:110, 73.3%) with 43 (39%) patients reporting annually having this vaccine. Thirty (20.4%) of the COPD patients had been vaccinated against pneumococcus (four of them had had a routine vaccination) while none of the asthmatic patients were vaccinated against pneumococcus.
Other actions taken by the patientsIn terms of the specific attitudes patients had to taking other preventative measures, asthma patients had taken significantly more actions in order to decrease their exposure to dust (n:107, 71.8% for asthma vs. n:24, 16.2%, for COPD, p<0.0001) and had used anti-mite bed covers (12.8% vs. 0%, p<0.0001). COPD patients, on the other hand, tended to use humidifiers more than asthma patients (26.8% for asthma vs. 38.7% for COPD, p<0.05). The use of special vacuum cleaners and air-conditioners was comparable in each group (p>0.05) (Table 2).
Patients with asthma (n:28, 18.8%) had had more pets in their home before diagnosis when compared to COPD patients (n:0, 0%). The majority (n=18; 64.3%) of these patients had sent the pet away following their asthma diagnosis.
There were no differences in terms of the actions each group took against triggers and behavioural changes in the ACT and MRC scores.
DiscussionThis cross-sectional study in a small subset of asthma and COPD patients revealed that both groups were similarly affected by certain triggers. However, their attitudes against some common triggers and behavioural changes in daily life did differ. Several areas in particular were seen more commonly according to the disease from which the patient was suffering; namely: choosing to vaccinate against influenza or pneumococcus was disease orientated; an attempt to humidify the environment was observed more in COPD; and more anti-allergic approaches, such as the use of strategies to decrease dust exposure, use of anti-mite bed sheets, and removal of pets from the home, as well as a change in analgesic preference after establishment of diagnosis were seen in patients with asthma.
In the current study, we compared the effect of triggers on symptoms in both diseases and interestingly showed that patients with both diseases were vulnerable to similar triggers and experienced symptoms to a similar degree. This finding is of particular importance since it is a common belief that the experiencing of symptoms upon exposure to triggering factors is more suggestive of asthma.1 However, the type of study design by exclusion of the cases in which a distinction between asthma and COPD cannot be made allowed us to make a reliable comparison between both groups, and thus, we were able to demonstrate that both patients with asthma and also those with COPD were vulnerable to certain triggers and developed symptoms following exposure. Thus, based on our findings, both groups of patients should be questioned about any possible links they may have between symptoms and triggering factors. At the same time, however, the fact that there may not be a distinctive positive response for asthma should also be kept in mind. This similarity in triggering factors might also reopen the debate on the Dutch hypothesis in which asthma and COPD are considered as a single entity whose pathogenesis involves similar environmental and host factors.16,17 Among these, airway hyperresponsiveness (AHR) was reported to be an important factor which makes the patient more susceptible to environmental stimuli.18 AHR is clearly triggered by specific allergen inhalation and/or non-specific factors, such as virus infections in asthma.10,11 However, factors that aggravate AHR in COPD are less well-documented, AHR is considered to be a risk factor in both the development and progression of COPD and asthma.19 There is a complex interaction between underlying hyperreactivity and clinical manifestations, and it has not yet been established whether the underlying mechanisms of AHR, more specifically those which predispose a person to asthma or COPD, are in fact one and the same.20 However, considering the fact that the shared triggers are responsible for the symptoms observed in both groups, it can be assumed that a similar mechanism could at least be partly responsible for underlying hyperreactivity in both diseases, although bronchial hyperreactivity was not evaluated in this study.
In this study, ACT scores of less than 24 displayed a predominance for some specific triggers, such as climbing stairs and doing housework, which were mainly reported by female patients, and emotional triggers. ACT scores of less than 24 corresponded to well-controlled (ACT scores: 20–24) or uncontrolled asthma (ACT scores less than 20).1,13 The fact that woman were particularly affected by physical triggers like climbing stairs and doing housework could be a reflection of insufficient control of the disease as these cases had current symptoms that bothered them and prevented them from participating in regular daily activities. Therefore, since this discomfort was fresher in their minds, they might have had an increased awareness of these triggers and therefore reported them with more frequency. However, there was no such relationship in the different MRC degrees of patients with COPD. As a result, we may assume that COPD patients are similarly sensitive to common triggers regardless of their MCR degree.
The identification of triggers is especially important when we design preventive actions against these triggers. Respiratory viral infections are recognised as the most frequent causes of asthma and COPD exacerbations among the preventable reasons for attacks.21,22 In accordance with the suggestion that inactivated influenza vaccinations be administered for moderate to severe asthma and COPD,1,2,23,24 we regularly recommend this vaccination to patients in our clinics. Pneumococcal polysaccharide vaccine is also recommended for COPD patients aged 65 years and older.25 Our findings on pneumococcal vaccination were also in accordance with this suggestion. On the other hand, even though influenza vaccination was for the most part preferred by COPD patients, the number of vaccinations was still lower than it should be in both groups. The reasons for this could be a lack of information and awareness, patients’ social conditions, and fear of injections.26 Thus, further strategies targeting patients’ individual concerns are needed in both groups to improve vaccination rates, particularly in the instance of influenza vaccination.
Cigarette smoking is a definite risk factor for the development of COPD.2 Moreover, exposure to smoking also induces symptoms in both asthma and COPD.1,2 Our study showed that approximately 10% of each group were still smoking, a lower figure than has previously been reported in our country.27 However, the most important outcome of this part of the study is the unsatisfactory number of cases who had tried to give up smoking, but failed, after the establishment of either diagnosis. These data once again emphasize the need for a more effective inclusion of smoking cessation strategies into the educational programmes of patients with asthma and COPD.
It is well known that aspirin and other COX-1 inhibitors can cause asthma attacks in susceptible patients, particularly in severe asthmatics with nasal polyps/chronic rhinosinusitis.28–31 In our study group, 11.4% of the asthmatics were aspirin sensitive. Asthma cases with aspirin sensitivity are recommended to use safer alternatives, such as paracetamol, a COX-3 inhibitor, and/or NSAIDs which preferentially inhibit COX2 inhibitors.30–32 However, in this study, regardless of whether or not there was underlying aspirin sensitivity, the majority of asthmatics preferred to use paracetamol. Some of these cases were also advised to use COX-2 inhibitors by their physicians after a negative drug provocation test. Interestingly, the majority of the patients with COPD also preferred to use paracetamol despite none of them having experienced an exacerbation following the use of any analgesics. The reason for COPD patients also preferring this medication, despite not being categorised as being at risk to sensitivity reactions, is of particular significance. Nevertheless, it is clear that whatever the underlying reason, the majority of cases with asthma and COPD in our study use paracetamol for the management of pain.
Of the other preventive actions that can be taken by patients, asthmatics reported employing more anti-allergic approaches, for instance taking actions to decrease exposure to dust, using anti-mite bedsheets, and the removal of pets from the home. Environmental control is a part of the treatment of asthmatic patients.1,33,34 The application of environmental control measures has been shown to reduce hospital admissions and improve symptoms in patients with asthma.33,34 Depending on the factors aggravating their symptoms, asthma patients are advised to perform these strategies by their doctors. On the other hand, COPD patients tended to perform limited environmental control strategies. Environmental control in the long-term management of COPD has recently been gaining more and more attention. When we consider the growing evidence that triggers seem to have as big a role in provoking symptoms in COPD as they do in asthma, it is logical to assume that strategies to control environmental factors will receive more and more attention from physicians in the long-term management of COPD.
In conclusion, our current findings on triggering factors in patients with asthma are consistent with previous data which demonstrated the importance of triggers in asthma symptoms. However, as a novel finding, our data drew attention to the fact that triggers also have a particular importance for COPD patients, an area in which very limited data is available. However, actions taken against some modifiable factors, such as vaccination status and attempts at smoking cessation, seem to be inadequate in both groups and need further supportive approaches. Therefore, particular attention must be paid to the detection of individual triggers and the development of strategies for controlling modifiable triggers during the education of both groups. However, an evaluation of the effect of these strategies on the prevention of disease progression, particularly in patients with COPD, is also required.
ContributorsThe authors had following contributions to the study:
Ömür Aydin: Study design, inclusion of asthma patients, data entrance, writing manuscript.
Gülfem Çelik: Study design, inclusion of asthma patients, statistical analysis, writing manuscript.
Zeynep Pınar Önen: Study design, inclusion of COPD patients, significant contribution to written manuscript.
İnsu Yilmaz: Study design, inclusion of asthma patients, significant contribution to written manuscript.
Seçil Kepil Özdemir: Study design, inclusion of asthma patients, significant contribution to written manuscript.
Öznur Yildiz: Study design, inclusion of COPD patients, significant contribution to written manuscript.
Dilşad Mungan: Study design, inclusion of asthma patients, significant contribution to written manuscript.
Yavuz Selim Demirel: Study design, inclusion of asthma patients, significant contribution to written manuscript.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.