The purpose of this study is to examine the changes in B lymphocyte subsets in patients receiving allergen immunotherapy.
MethodsB lymphocyte subsets of patients before immunotherapy and one year after immunotherapy began were examined using the flow cytometric method. Age-matched healthy children served as the control group.
ResultsTwenty-two patients with asthma and/or allergic rhinitis and 14 healthy, age-matched controls were included in the study. The median age of the patients was 13 years old (range: 6–20 years), and eleven (50.0%) were male. The median age of the healthy controls was also 13 years old (range: 7–17), and seven (50.0%) were male. In the age group from 11 to 15 years; the patients’ relative and absolute counts of active and mature sensitive B cells were higher than those of the healthy children (p=0.027–0.012 and p=0.032–0.010, respectively) before immunotherapy. The relative and absolute counts of active B cells before immunotherapy were also significantly higher than those of after immunotherapy (p=0.001–0.001, p=0.025–0.037, and p=0.029–0.035, respectively). Before immunotherapy, the relative and absolute counts of mature sensitive B cells were significantly higher than those obtained after immunotherapy (p=0.024–0.006) in the 11–15-year-old age group.
ConclusionsAllergen immunotherapy directly influences B cell differentiation and causes a decrease in the count of active B cells. This finding is relevant because the B cell count can be used as a guide in the assessment of an individual patient's treatment response and also when determining whether to continue the immunotherapy.
During sensitisation to an allergen, the priming of allergen-specific T helper 2 (Th2) cells results in the production of Th2 cytokines (interleukin-4 (IL-4) and IL-13)), which are responsible for class switching to the ¿ immunoglobulin heavy chain and allow IgE production by B cells. IgE sensitises mast cells and basophils by binding with the high-affinity receptor for IgE (Fc¿RI), which is expressed on the surface of these cells. When the allergen crosslinks with the IgE-Fc¿RI complexes, mast cells and basophils degranulate, releasing vaso-active amines (mainly histamine), lipid mediators, chemokines, and other cytokines, all of which characterise the immediate phase of the allergic reaction. IgE also binds with Fc¿RI on the surface of dendritic cells and monocytes, as well as to the low-affinity receptor for IgE, Fc¿RII (also known as CD23), on the surface of B cells. This process increases the uptake of the allergen by these antigen-presenting cells and the subsequent presentation of allergen-derived peptides to specific CD4+ T cells, which drive the late phase of the allergic reaction. Allergen immunotherapy (AIT) is associated with improved tolerance to allergen challenges as well as a decrease in immediate-phase and late-phase allergic inflammation.1
The possible mechanisms of AIT include a reduction in mast cell reactivity, decreases in basophil responses, a drop in the specific immunoglobulin IgE, increases in IgG4, and induction of regulatory T cells.2 Recently, a new subset of B cells has been identified as regulatory (Bregs) due to its capacity to secrete interleukin-10 (IL-10); these B cells likely play a role in the mechanism of AIT.3 CD23 is located on B cells and has been proposed to participate in both positive and negative feedback mechanisms in the regulation of IgE synthesis.4 However, there is a lack of information about the role of B cell subsets and CD23 (Fc¿RII) in AIT mechanisms.
The purpose of this study is to examine the changes in B lymphocyte subsets in patients receiving AIT.
Materials and methodsStudy populationThis study included 22 patients and 14 age-matched, healthy control children. The patients and the healthy children were divided into three groups as follows: 6–10 years, 11–15 years, and children >15 years of age. B lymphocyte subsets of the age-matched healthy children and the patients were assessed both before and one year after a monthly AIT treatment. The patients were also assessed using an asthma symptom score (cough, wheezing, dyspnoea, chest pain) and an allergic rhinitis symptom score (sneezing, itchy nose, runny nose, and nasal blockage) both before and after AIT. The association between the symptoms and sleep and daily activities was also examined. In addition, patients with allergic rhinitis were assessed using a visual analogue score.5–7
Asthma symptom scores (cough, wheezing, dyspnoea, chest pain)
0 p: No complaints during either the night or the day
1 p: Complaints do not affect daily work or sleep
2 p: Complaints have a light effect on daily work and sleep
3 p: Complaints affect daily work and sleep on more than two days per week
Note: Chest pain was not evaluated because it is rare in children.
Allergic rhinitis symptom score (sneezing, itchy nose, runny nose, and nasal blockage)
0 p: No complaints during either the night or the day
1 p: Complaints are present but are not disturbing
2 p: Complaints are disturbing but do not affect daily work or sleep
3 p: Complaints affect both daily work and sleep
Laboratory studiesVenous blood samples 3ml in volume were obtained from each participant using tubes containing ethylenediamine tetraacetic acid (EDTA). Immunophenotyping was performed with the following monoclonal antibodies: IgD PE, CD19 APC, and CD27 FITC (BD Biosciences, Pharmingen, Germany). The relatives of the B lymphocyte subsets were analysed using flow cytometry (BD FACS Calibur; BD Biosciences, San Jose, CA, USA). The peripheral CD19+ B cell subsets were defined as follows: active (CD19+CD38−CD21−), memory (CD19+CD27+), naive (CD19+IgD+CD27−), mature (CD19+IgD−CD27+), mature and sensitive (CD19+IgD−CD23+), mature and insensitive (CD19+IgD−CD23−), naive and sensitive (CD19+IgD+CD23+), naive and insensitive (CD19+IgD+CD23−), non-switched (CD19+ IgD+ CD27+), and class-switched memory B cells as CD19+IgD−CD27+.8,9 CD23-positive B cells were considered sensitive, while CD23-negative B cells were labelled insensitive.
For the atopy assessment, a skin prick test was performed. Histamine (10mg/ml) was used as a positive control in the skin prick test, and physiological serum was used as the negative control. The size of the wheal was measured 15–20min after the allergen had been applied to the skin. According to the physiological serum response, a wheal of 3mm or greater was considered significant.10 The following allergens were used in the skin prick test: tree mix (Castanea vulgaris, Quercus robur, Fagus sylvativ ACE), grass mix (Anthoxant odoratum, Dactylis glomerata, Lolium pere me, Phleum protein France, Poa pratensis), weeds (Chenopodium album, Amaranthus retrolexus), Dermatophagoides farinea, Dermatophagoides pteronyssinus (Stallergenes SA, 92160 Antony, France).
Statistical analysesAll statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS for Windows, version 15). All data were expressed as the median or relatives that were caused by distributions that were not considered normal. A Mann–Whitney U-test was used, and values of p<0.05 were accepted as statistically significant.
Ethical disclosureEthical approval was granted in decision no. OMU KAEK 2013/347 by Ondokuz Mayis University's Ethics Committee of Medical Research.
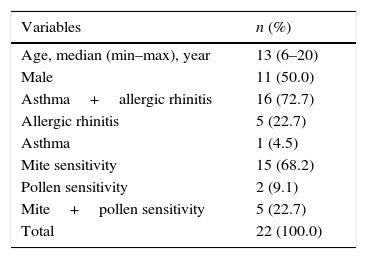
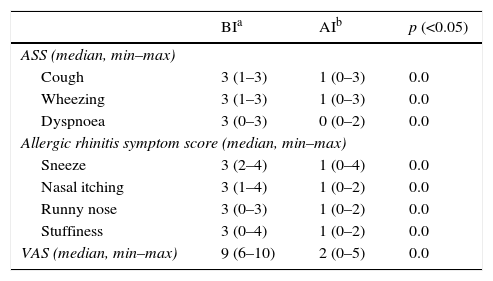
ResultsThe median age of the 22 patients and 14 healthy controls included in this study was 13 years (range: 6–20), and 11 (50.0%) were male. The median age of the healthy controls was also 13 years (range: 7–17), and seven (50.0%) were male. Seventeen patients (77.2%) were diagnosed with asthma, while 21 (95.4%) had allergic rhinitis. Fifteen (68.2%) patients were found to have mite sensitivity, and two (9.1%) had a pollen sensitivity; five (22.7%) patients suffered from both a mite and a pollen sensitivitiy (Table 1). After one year of AIT, the asthma symptom scores, allergic rhinitis visual analogue scales, and allergic rhinitis symptom scores of the patients had made a significant recovery compared with those before AIT (p=0.0) (Table 2).
Demographics of patients treated with allergen immunotherapy.
| Variables | n (%) |
|---|---|
| Age, median (min–max), year | 13 (6–20) |
| Male | 11 (50.0) |
| Asthma+allergic rhinitis | 16 (72.7) |
| Allergic rhinitis | 5 (22.7) |
| Asthma | 1 (4.5) |
| Mite sensitivity | 15 (68.2) |
| Pollen sensitivity | 2 (9.1) |
| Mite+pollen sensitivity | 5 (22.7) |
| Total | 22 (100.0) |
Comparisons of patient symptoms before immunothreapy and after immunotherapy.
| BIa | AIb | p (<0.05) | |
|---|---|---|---|
| ASS (median, min–max) | |||
| Cough | 3 (1–3) | 1 (0–3) | 0.0 |
| Wheezing | 3 (1–3) | 1 (0–3) | 0.0 |
| Dyspnoea | 3 (0–3) | 0 (0–2) | 0.0 |
| Allergic rhinitis symptom score (median, min–max) | |||
| Sneeze | 3 (2–4) | 1 (0–4) | 0.0 |
| Nasal itching | 3 (1–4) | 1 (0–2) | 0.0 |
| Runny nose | 3 (0–3) | 1 (0–2) | 0.0 |
| Stuffiness | 3 (0–4) | 1 (0–2) | 0.0 |
| VAS (median, min–max) | 9 (6–10) | 2 (0–5) | 0.0 |
ASS: asthma symptom score, VAS: visual analogue scale.
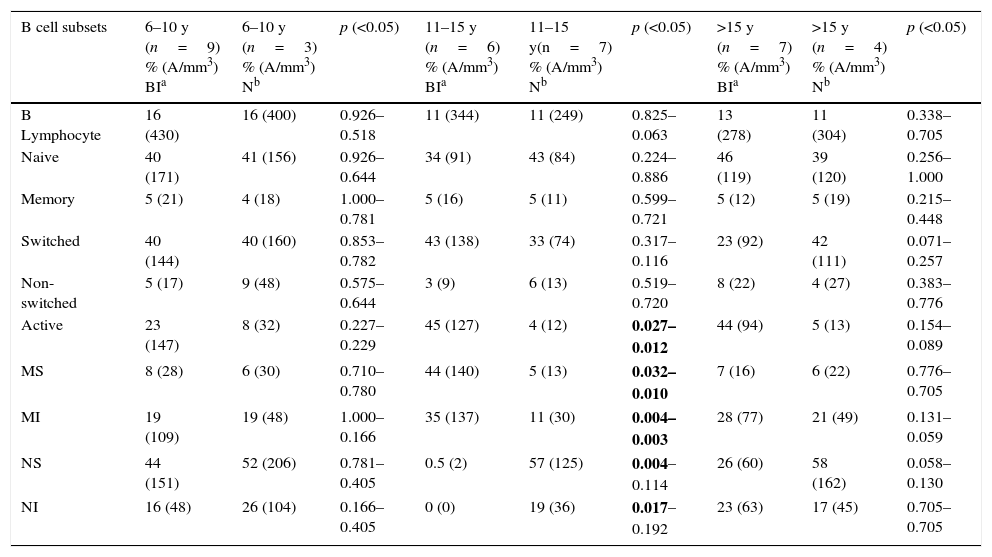
When the patients’ B lymphocyte subsets before AIT were compared with those of healthy children, no significant relative or absolute differences were identified between the two groups in terms of naive, memory, switched, and non-switched B cells (p>0.05). In the age group from 11 to 15, the relative and absolute numbers of active B and mature sensitive cells before AIT were higher than those of the healthy children (p=0.027–0.012 and p=0.032–0.010, respectively). In the same age group, the relative number of naive sensitive B cells in the atopic patients was lower than the number in the healthy controls (p<0.04). When children from 6 to 10 years and >15 years of age were compared, no significant difference was found in terms of B lymphocyte subsets (p>0.05) (Table 3).
Comparison of B lymphocyte subsets of atopic children and healthy children.
| B cell subsets | 6–10 y (n=9) % (A/mm3) BIa | 6–10 y (n=3) % (A/mm3) Nb | p (<0.05) | 11–15 y (n=6) % (A/mm3) BIa | 11–15 y(n=7) % (A/mm3) Nb | p (<0.05) | >15 y (n=7) % (A/mm3) BIa | >15 y (n=4) % (A/mm3) Nb | p (<0.05) |
|---|---|---|---|---|---|---|---|---|---|
| B Lymphocyte | 16 (430) | 16 (400) | 0.926–0.518 | 11 (344) | 11 (249) | 0.825–0.063 | 13 (278) | 11 (304) | 0.338–0.705 |
| Naive | 40 (171) | 41 (156) | 0.926–0.644 | 34 (91) | 43 (84) | 0.224–0.886 | 46 (119) | 39 (120) | 0.256–1.000 |
| Memory | 5 (21) | 4 (18) | 1.000–0.781 | 5 (16) | 5 (11) | 0.599–0.721 | 5 (12) | 5 (19) | 0.215–0.448 |
| Switched | 40 (144) | 40 (160) | 0.853–0.782 | 43 (138) | 33 (74) | 0.317–0.116 | 23 (92) | 42 (111) | 0.071–0.257 |
| Non-switched | 5 (17) | 9 (48) | 0.575–0.644 | 3 (9) | 6 (13) | 0.519–0.720 | 8 (22) | 4 (27) | 0.383–0.776 |
| Active | 23 (147) | 8 (32) | 0.227–0.229 | 45 (127) | 4 (12) | 0.027–0.012 | 44 (94) | 5 (13) | 0.154–0.089 |
| MS | 8 (28) | 6 (30) | 0.710–0.780 | 44 (140) | 5 (13) | 0.032–0.010 | 7 (16) | 6 (22) | 0.776–0.705 |
| MI | 19 (109) | 19 (48) | 1.000–0.166 | 35 (137) | 11 (30) | 0.004–0.003 | 28 (77) | 21 (49) | 0.131–0.059 |
| NS | 44 (151) | 52 (206) | 0.781–0.405 | 0.5 (2) | 57 (125) | 0.004–0.114 | 26 (60) | 58 (162) | 0.058–0.130 |
| NI | 16 (48) | 26 (104) | 0.166–0.405 | 0 (0) | 19 (36) | 0.017–0.192 | 23 (63) | 17 (45) | 0.705–0.705 |
MS: mature and sensitive, MI: mature and insensitive, NS: naive and sensitive, NI: naive and insensitive, A: absolute.
Bolded P-values indicate statistical significance.
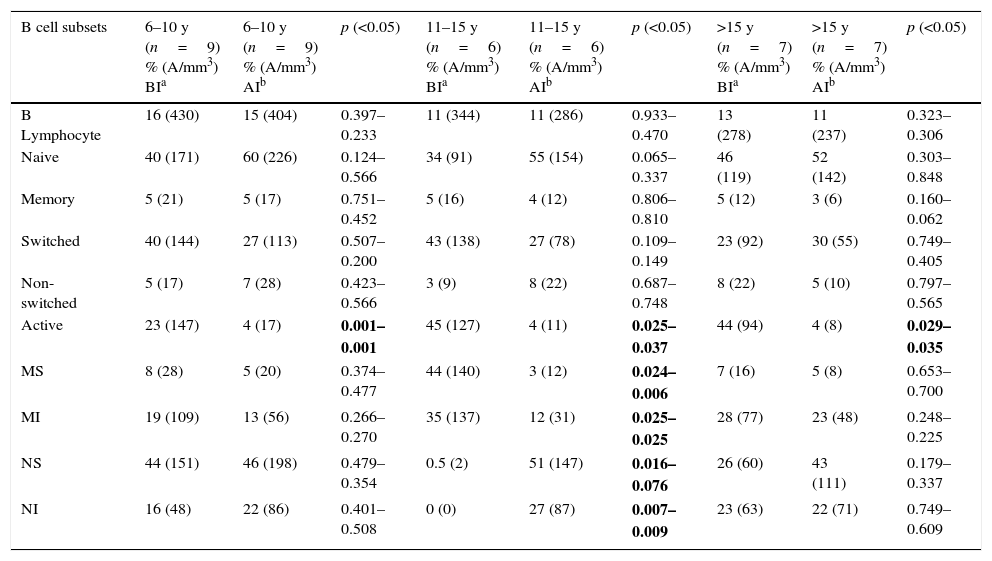
When the before AIT and after AIT (one year later) B lymphocyte subsets of the patients were compared, no significant relative or absolute count difference was found between the 6–10, 11–15, or >15-year-old groups in terms of B lymphocyte, naive, memory, switched, and non-switched cells (p>0.05). The relative and absolute counts of active B cells before AIT were significantly higher than those obtained after AIT in all participant age groups (6–10 years: p=0.001–0.001, 11–15 years: 0.025–0.037, and >15 years: 0.029–0.035). In the group from 11 to 15 years, the relative and absolute numbers of mature sensitive cells were higher before AIT compared with those after AIT (p=0.024–0.006). The relative and absolute counts of naive sensitive cells in the 11–15 year-old age group were lower before AIT than they were after AIT (p=0.024–0.006). In the comparison of 6–10-year-old and >15-year-old age groups, no significant difference was found between groups in terms of mature and sensitive and naive and sensitive B cells (p>0.05) (Table 4).
Comparison of B lymphocyte subsets of patients before immunotherapy and after immunotherapy.
| B cell subsets | 6–10 y (n=9) % (A/mm3) BIa | 6–10 y (n=9) % (A/mm3) AIb | p (<0.05) | 11–15 y (n=6) % (A/mm3) BIa | 11–15 y (n=6) % (A/mm3) AIb | p (<0.05) | >15 y (n=7) % (A/mm3) BIa | >15 y (n=7) % (A/mm3) AIb | p (<0.05) |
|---|---|---|---|---|---|---|---|---|---|
| B Lymphocyte | 16 (430) | 15 (404) | 0.397–0.233 | 11 (344) | 11 (286) | 0.933–0.470 | 13 (278) | 11 (237) | 0.323–0.306 |
| Naive | 40 (171) | 60 (226) | 0.124–0.566 | 34 (91) | 55 (154) | 0.065–0.337 | 46 (119) | 52 (142) | 0.303–0.848 |
| Memory | 5 (21) | 5 (17) | 0.751–0.452 | 5 (16) | 4 (12) | 0.806–0.810 | 5 (12) | 3 (6) | 0.160–0.062 |
| Switched | 40 (144) | 27 (113) | 0.507–0.200 | 43 (138) | 27 (78) | 0.109–0.149 | 23 (92) | 30 (55) | 0.749–0.405 |
| Non-switched | 5 (17) | 7 (28) | 0.423–0.566 | 3 (9) | 8 (22) | 0.687–0.748 | 8 (22) | 5 (10) | 0.797–0.565 |
| Active | 23 (147) | 4 (17) | 0.001–0.001 | 45 (127) | 4 (11) | 0.025–0.037 | 44 (94) | 4 (8) | 0.029–0.035 |
| MS | 8 (28) | 5 (20) | 0.374–0.477 | 44 (140) | 3 (12) | 0.024–0.006 | 7 (16) | 5 (8) | 0.653–0.700 |
| MI | 19 (109) | 13 (56) | 0.266–0.270 | 35 (137) | 12 (31) | 0.025–0.025 | 28 (77) | 23 (48) | 0.248–0.225 |
| NS | 44 (151) | 46 (198) | 0.479–0.354 | 0.5 (2) | 51 (147) | 0.016–0.076 | 26 (60) | 43 (111) | 0.179–0.337 |
| NI | 16 (48) | 22 (86) | 0.401–0.508 | 0 (0) | 27 (87) | 0.007–0.009 | 23 (63) | 22 (71) | 0.749–0.609 |
MS: mature and sensitive, MI: mature and insensitive, NS: naive and sensitive, NI: naive and insensitive, A: absolute.
Bolded P-values indicate statistical significance.
AIT involves the repeated administration of the sensitising allergen by subcutaneous injection or sublingual application. AIT is disease modifying and has a duration of effect beyond the treatment period. In addition, AIT prevents the development of new allergic sensitisations and reduces the progression of allergic rhinitis to asthma. It is known that the ratio of T helper 1 (Th1) cytokines to Th2 cytokines is increased following AIT, and functional regulatory T cells are induced during AIT. The production of IL-10 by monocytes, macrophages, B cells, and T cells is also increased. The expression of transforming growth factor-β (TGFβ) rises; along with IL-10, TGFβ might contribute to regulatory T-cell function and immunoglobulin class switching to IgA, IgG1 and IgG4. These immunoglobulins compete with IgE for allergen binding, decreasing the rate of allergen capture and presentation that is facilitated by IgE in complex with the high-affinity receptor for IgE (Fc¿RI) or the low-affinity receptor for IgE (Fc¿RII). In addition, AIT reduces the number of mast cells and the ability of mast cells to release mediators. The recruitment of eosinophils and neutrophils to sites of allergen exposure is also reduced.1
In this study, we examined the changes in B lymphocytes of patients receiving AIT using the flow cytometric method. B lymphocytes undergo a complex maturation process in the bone marrow (pro-B cell, pre-B cell, immature cell, and mature B cell).11 Mature naive B cells that have completed their growth and maturation in the bone marrow migrate to peripheral lymphoid cells and wait to encounter antigens. Mature naive B cells can recognise the antigen; however, they do not secrete antibodies. The complex process of B cell activation is required for antibody secretion.12 B cell activation is either T cell dependent or T cell independent.13 Protein antigens cause T cell dependent B cell activation. For protein antigens, the following steps are involved: (1) Recognition of antigens by B cells and initiation of the intracellular signalling cascade; (2) Antigen presentation by B cells to Th cells; (3) B cell activation with the help of Th cells; (4) B cell differentiation (development of antibody-releasing effector B cells, heavy chain isotype/class switching, affinity maturation, and development of memory B cells); (5) Elimination of antigens by the release of antibodies; and (6) Homeostasis (termination of the humoral immune response).12
Mature B cells express CD23 and B cell receptors. AIT activates CD23 to produce IL-10 in mature B cells at lower doses. Exposure to specific antigens at optimal doses can activate mature B cells to produce IL-10 and therefore suppress the skewed antigen-specific Th2 responses.14 In this study, there was no difference between the patients and the healthy children in terms of naive, memory, switched, and non-switched B lymphocyte cells. In the 11–15 age group, active B and mature B (both sensitive and insensitive) lymphocyte levels were higher before AIT than those of the control group in terms of their relative and absolute counts. A significant decrease was found in the count of naive B lymphocytes following AIT (both sensitive and insensitive) when compared with the control group in this same age group. In other age groups, no statistically significant difference was found between the patients and the healthy participants in terms of active, mature, or naive B cells. The different results obtained between age groups may be due to the limited number of patients and controls in our study. Higher numbers of pre-immunotheraphy mature B and active B lymphocytes in patients indicate that the antigen-dependent differentiation of B lymphocytes should be faster in allergic inflammation. Natural exposure to a relevant allergen is often associated with an increase in IgE synthesis. AIT often induces a transient increase in serum-specific IgE levels followed by a gradual decrease over months or years of continued treatment.15 Active B cells also probably play a significant role in allergic reactions and turn into effector cells in IgE cells and influence antigen-specific IgE production.
In this study, there were no differences between the relative and absolute counts, naive, memory, switched, and non-switched B lymphocytes either before AIT or after AIT. However, in all age groups, the relative and absolute counts of active B cells showed a significant decrease following AIT. In addition, in the 11–15 age group, a decrease was found in mature B lymphocytes (both sensitive and insensitive) with AIT. In the same age group, there was an increase in the number of naive B lymphocytes before AIT. These data indicate that AIT may produce its main effect by decreasing B cell activation. In addition, a decrease in mature B (both sensitive and insensitive) lymphoctyes and a rise in naive B (both sensitive and insensitive) lymphocytes compared with the numbers before AIT indicate that AIT is also effective on the other steps of B cell differentiation that are induced by allergens.
Patients who receive AIT should be assessed every 6–12 months.16 Our patients were clinically followed using their asthma symptom, allergic rhinitis symptom, and visual analogue scale scores. In the current study, the number of active B cells was higher in atopic children when compared with healthy children before AIT. Patients with B cell activation report many symptoms. After the first year of AIT, a significant decrease was seen in the active B lymphocyte count in these patients.
In conclusion, each time atopic children encounter an antigen, B cell activation increases with the help of T cells, which increase antigen-specific IgE oscillation. It is thought that the significant decrease in active B lymphocytes after AIT can be a guide in assessing the treatment response and in reviewing the decision of whether to continue treatment.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestNone of the authors has any conflict of interest, financial or otherwise.