There are few studies in children on the natural course of chronic spontaneous urticaria (CSU) because of its relative infrequency in childhood.
ObjectiveTo estimate the rate of remission and evaluate the prognostic factors in children with CSU.
MethodA total of 52 children with CSU were prospectively followed over a period of three years.
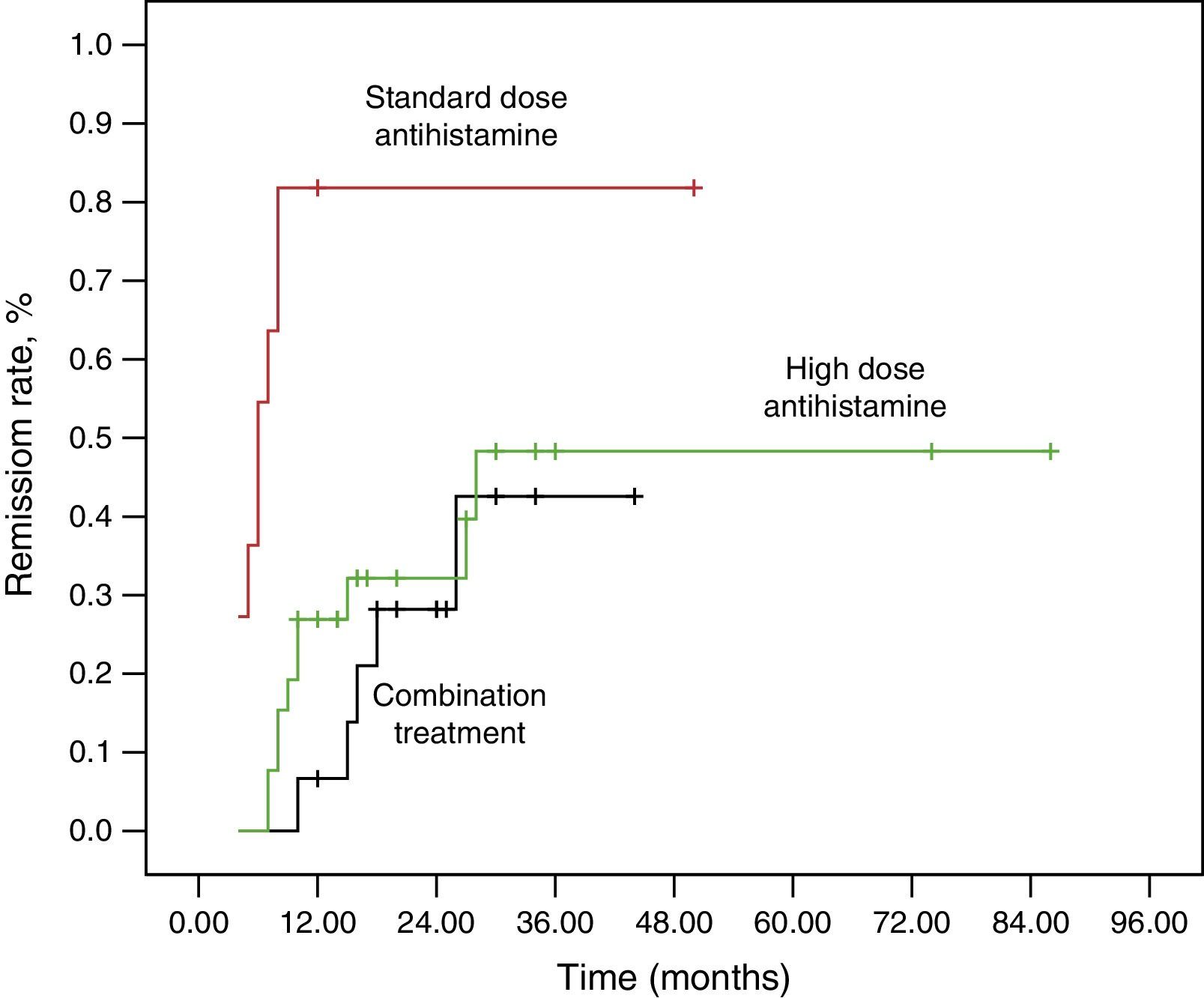
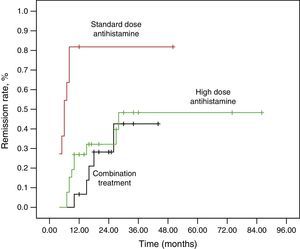
ResultsThe remission rates at 12 months and 36 months were 32.7% and 48.1%. The mean duration of disease at the first visit in the non-remission group was higher than in the remission group at the end of the study (P=0.016). The remission rate of the patients who had been treated by standard dose antihistamine was higher than that of the patients who had been treated with the high-dose antihistamine and combination medications (P=0.004, P<0.001). The treatment steps were independent prognostic factors for remission by logistic regression analysis.
ConclusionOur study indicates that urticaria controlled by a standard dose of antihistamine can predict a good prognosis independently from disease duration at first visit.
Chronic urticaria (CU) in childhood is a rare and challenging disease for both patients and doctors because it is a long-term disease and there are limited data about its natural course. It is characterised by short-lived itchy wheals occurring at least twice a week and persisting for six weeks or longer.1–3 The causative factors, such as physical factors, food allergy, drug reaction, connective tissue disease, chronic infection, and parasitic infestation are identified in <30–40% of patients with CU.4,5 The diagnosis of chronic idiopathic urticaria (CIU) is made when no apparent trigger or other cause is identified. Although accurate data on the prevalence of CSU are unavailable in children, this condition is thought to affect 0.1–3% of adults in the United States and Europe.1 The presence of histamine-releasing IgG anti-FcRI and anti-IgE autoantibodies suggests an autoimmune pathogenesis of disease in a subset of patients; 31–47% of children with CSU have an autoimmune cause with a positive autologous serum skin test (ASST).6–9
CSU is an unpredictable disease. There is limited literature regarding the prognosis of CSU, and most relates to adults.10–12 Only a few studies on the natural course of the disease have been performed on children.6,8,13,14 Although some studies on adults have suggested that a positive ASST result, the presence of angio-oedema and positive anti-thyroid antibodies might indicate a long disease duration, we still know little about the association between the natural course and the clinical/laboratory parameters of CSU in children.11
The aim of this prospective study was to investigate the natural course and prognostic variables of CSU in childhood.
MethodsThe study was performed in the Department of Paediatric Allergy and Immunology at Kocaeli University, Turkey, from January 2012 to January 2015. The study included children with urticaria present daily or almost daily for duration of at least 6 weeks.
At the screening visits, all patients underwent a detailed history, and physical examination. In all instances, personal history of atopic diseases (such as atopic dermatitis, asthma, allergic rhinitis), any drug intake, presence of associated angio-oedema, signs of infection (such as upper airway infection, urinary tract infection, fever), signs of connective tissue disease and auto-inflammatory syndromes (such as arthritis, arthralgia, fever, conjunctivitis, muscle and skin tenderness after exposure to cold, sensorineural hearing loss), trigger of urticaria (such as cold, exercise, hot shower), and recent travel history were investigated. In addition, family history of urticaria, atopic disease and autoimmune conditions were recorded. Skin prick tests with commercial allergens (Dermatophagoides pteronyssinus, Dermatophagoides farina, grass, tree and weed pollens, Alternaria, Penicillium and Aspergillus, dog and cat epithelia, milk, egg, soy, peanut, hazelnut, fish, wheat) and provocation tests for physical urticaria were performed on patients with a suggestive history.
To investigate the known causes of CU, the patients underwent the following laboratory workup: (i) complete blood cell count, blood chemistry, erythrocyte sedimentation rate, liver function tests; (ii) infective panel (serologic assays for hepatitis A and B virus, Epstein–Barr virus (EBV), cytomegalovirus (CMV), urine analysis and culture, throat culture, H. pylori IgG antibodies and three stool examinations for parasites); (iii) autoimmune panel (anti-nuclear antibody (ANA), anti-thyroglobulin (anti-TG) antibody, anti-thyroperoxidase (anti-TPO) antibody, free T4, thyroid-stimulating hormone).
At the first visit, the patients without a clearly defined cause were enrolled based on exclusion criteria that included isolated physical urticaria, atopic dermatitis, infectious diseases, connective tissue diseases, auto-inflammatory syndromes and food hypersensitivity. Informed consent was obtained from the parents. ASSTs were performed on all patients. The patients were followed up every four weeks. After follow-up at least one year, the patients were allowed to leave the study if they had been in remission.
Autologous serum skin testThe patients discontinued short-acting antihistamines for at least three days. Briefly, 0.05ml of sterile, fresh, undiluted autologous serum or 0.9% sterile saline as a negative control was injected intradermally into the volar aspect. A skin prick test with histamine (10μg/ml) was used as a positive control. Wheal and flare reactions were recorded after 30min. An ASST was considered positive when the wheal diameter was 1.5mm or greater compared with that elicited by saline.
Urticaria activity scoreDisease activity was assessed with an urticaria activity score (UAS). Daily assessments for key urticarial symptoms were recorded by the patients, or parents if patients were young, wheal number (range 0–3) and pruritus intensity (range 0–3), which were added together (range 0–6). The UAS7 is the sum of the scores from seven consecutive days (maximum 0–42).
TreatmentThe second-generation H1-antihistamine at the standard dose for age was initiated in the patients who did not receive the H1-antihistamine treatment (step 1). The dose increased up to fourfold if urticaria was not controlled (step 2). If urticaria was not controlled, combination therapy was used including high-dose antihistamine plus montelukast and/or ranitidine (step 3). The dose increased or combined with montelukast and/or ranitidine for patients treated with the second-generation H1-antihistamines already at the referral time. Remission was considered if symptoms did not recur for at least three months without drugs.
Statistical analysisAll statistical analyses were performed using IBM SPSS for Windows version 20.0 (SPSS, Chicago, IL, USA). Kolmogorov–Smirnov tests were used to test the normality of data distribution. Continuous variables were expressed as means±standard deviation or median (interquartile range), and categorical variables were expressed as counts (percentages). Comparisons of continuous variables between the groups were performed using the Student t test and the Mann–Whitney U test. Comparisons of categorical variables between the groups were performed using the Pearson, Fisher, Yates, Monte Carlo χ2 test. Logistic regression analysis was used to assess the independent association between the factors that differed significantly in the remission and non-remission groups based on univariate analysis. A two-sided P value <0.05 was considered statistically significant.
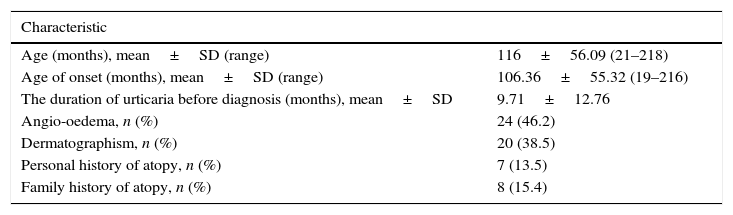
ResultsDemographic dataAmong 60 patients screened, five patients were lost to follow-up within the first year, and three patients were excluded from the study because they had poor drug compliance. The 52 patients were followed up for at least one year. Demographic and clinical features of the patients are shown in Table 1. The ASST gave a positive result in 23 of 52 children (44.2%) with CSU. There was no difference in the demographic data, laboratory investigations and UAS between ASST-positive and ASST-negative patients. In all, six children (11.5%) had positive anti-thyroid antibodies (four patients (7.7%) anti-TPO and two patients (3.8%) anti-TG) and ten (19.2%) patients had positive ANA titres. No patients had abnormal thyroid function test results. Seven patients (13.5%) had atopic diseases (asthma 9.6%, allergic rhinitis 3.9%) and eight children (15.4%) had family history of allergic disease. No patients had family history of chronic urticaria.
Demographic and clinical features of the patients.
| Characteristic | |
|---|---|
| Age (months), mean±SD (range) | 116±56.09 (21–218) |
| Age of onset (months), mean±SD (range) | 106.36±55.32 (19–216) |
| The duration of urticaria before diagnosis (months), mean±SD | 9.71±12.76 |
| Angio-oedema, n (%) | 24 (46.2) |
| Dermatographism, n (%) | 20 (38.5) |
| Personal history of atopy, n (%) | 7 (13.5) |
| Family history of atopy, n (%) | 8 (15.4) |
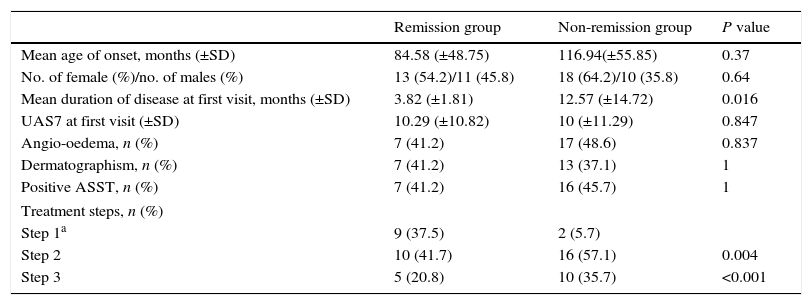
The remission rate was 32.7% (n:17) at one year of their follow-up in the 52 patients. The cumulative remission rate was 46.1% at three years (at the end of study). The clinical and laboratory profiles of the remission and non-remission groups at the end of study are summarised in Table 2. The patients in the remission group were compared with those in the non-remission group and there were no significant differences in terms of age, gender, UAS, ASST positivity and the presence of dermographism and/or angio-oedema.
The clinical and laboratory profiles of the remission and non-remission groups at three years (at the end of study).
| Remission group | Non-remission group | P value | |
|---|---|---|---|
| Mean age of onset, months (±SD) | 84.58 (±48.75) | 116.94(±55.85) | 0.37 |
| No. of female (%)/no. of males (%) | 13 (54.2)/11 (45.8) | 18 (64.2)/10 (35.8) | 0.64 |
| Mean duration of disease at first visit, months (±SD) | 3.82 (±1.81) | 12.57 (±14.72) | 0.016 |
| UAS7 at first visit (±SD) | 10.29 (±10.82) | 10 (±11.29) | 0.847 |
| Angio-oedema, n (%) | 7 (41.2) | 17 (48.6) | 0.837 |
| Dermatographism, n (%) | 7 (41.2) | 13 (37.1) | 1 |
| Positive ASST, n (%) | 7 (41.2) | 16 (45.7) | 1 |
| Treatment steps, n (%) | |||
| Step 1a | 9 (37.5) | 2 (5.7) | |
| Step 2 | 10 (41.7) | 16 (57.1) | 0.004 |
| Step 3 | 5 (20.8) | 10 (35.7) | <0.001 |
We found that the duration of disease at the first visit in the non-remission group was higher than that in the remission group (P=0.016). In all, 11 patients (21.2%) and 26 patients (50%) were prescribed standard and high-dose antihistamine. Fifteen patients (28.8%) were prescribed combination medications. The remission rate at three years in patients who were taking only standard dose antihistamine was higher than in those taking high-dose antihistamine and combination medications for control of urticaria (standard dose–high-dose antihistamine, P=0.004; standard dose antihistamine–combination therapy, P<0.001). Logistic regression analysis, which included disease duration at first visit and medication usage, showed that treatment steps were independent prognostic factors of remission (standard dose antihistamine–high-dose antihistamine: odds ratio (OR), 0.134; 95% confidence interval (CI), 0.021–0.869; P=0.035, standard dose antihistamine–combination therapy: OR, 0.106; 95% CI, 0.014–0.788; P=0.028). A Kaplan–Meier curve was produced to determine the remission rate for the treatment groups (Fig. 1).
There were no differences in remission rates and medication requirements between ASST-positive and ASST-negative patients.
The association between the clinical/laboratory parameters and UAS7 was analysed. Anti-TPO-positive patients had higher urticaria scores compared with patients without antibody (P=0.018). The presence of dermographism and/or angio-oedema, ASST positivity and ANA did not influence the UAS.
DiscussionThe early identification of prognostic factors that can predict the course of the disease is desirable because CSU is a long-term disease and is a challenge for the patient and the doctor in most cases. In our prospective study, we investigated the remission rate and several parameters as predictors of the natural course in children with CSU.
The remission rates in this study were 32.7% and 46.1% at one and three years, respectively. The remission rate at one year was much higher than has been reported previously. Sahiner et al.8 reported remission from urticaria in 16.5% of children after one year. After three years, 38.8% of the children were urticaria free and after five years, it had resolved in half of the cases. Chansakulporn et al.14 found that 18.5%, 54% and 67.5% of patients were in remission at one, three, and five years from the onset of symptoms, respectively.
Previous studies on adult populations demonstrated that the remission rate decreases as urticaria persists.10,15 In our study, the median duration of urticaria before inclusion was 4.5 months (range, 2–60 months), and the patients had short disease duration compared with those in a paediatric study reported by Chansakulporn et al.14 (2.7 years; range, 0.3–11.3 years). This factor may account for the high remission rate at one year. Although on univariate analysis the mean duration of urticaria before inclusion and the treatment steps in the remission and non-remission groups were significantly different in our study, no statistically significantly differences were found in disease duration by logistic regression analysis.
The remission rate of the patients who had taken standard dose antihistamine was higher than that of patients who had been treated with high-dose antihistamine or combination medications in logistic regression analysis. There were no significant differences in UAS between the remission and non-remission groups. In this study, we clarified that the response to standard dose antihistamine was an important prognostic factor for remission regardless of the activity of urticaria and the disease duration at first visit. This is consistent with a previous report by Hiragun et al.12
Although adult studies have emphasised that a positive ASST result might indicate increased disease activity and/or long disease duration,10,16 our study showed no significant difference between children who were ASST positive and ASST negative with regard to clinical future, UAS and medication usage. ASST-positive patients also had the same remission rate as ASST-negative patients. A positive ASST result did not determine a poorer natural course. This is consistent with the results of other researchers.14
We investigated whether the presence of angio-oedema affects the remission rate of disease. Our study revealed that angio-oedema was not related to the prognosis, in contrast to previous reports.17,18
Our study did not demonstrate any effect of gender on the remission rate of CSU. This was in contrast to the retrospective study of Harris et al.,13 who found that girls had a higher rate of remission. Sahiner et al.18 reported that the prognosis was unfavourable in girls older than 10 years; on multivariate analysis, age, gender, the presence of angio-oedema or other allergic diseases, autoimmunity family history, ASST positivity or abnormal laboratory results did not predict the prognosis.
The association of CSU with autoimmune thyroid disease has frequently been reported. In agreement with other reports, we demonstrated an 11.5% prevalence of thyroid autoantibodies in children with CSU.19,20 This was significantly higher than in healthy children. There were no significant differences between anti-TG and/or anti-TPO negative and positive patients in remission rates or in response to medications, but patients with positive anti-TPO had significantly higher chronic activity scores at first visit than patients with negative anti-TPO. Caminiti et al.21 hypothesised that children with active chronic urticaria may have associated autoimmune conditions more frequently. This result suggests that children with higher disease activity may have anti-TPO antibodies more frequently; a long-term follow-up would be important, to identify any association between CSU and autoimmune thyroid disease in children because autoimmune diseases develop over several years.
The small sample size is a limitation of our study. When considering the low prevalence of CU in childhood, it seems difficult to reach a sufficient size of sample in a single centre. Although three patients were excluded from the present study because of poor compliance, patients’ compliance to the treatment was not evaluated in detail, which could be considered another limitation. Further multicentre, prospective studies which contain a larger sample size and evaluation of treatment compliance based on objective measures are still needed.
In conclusion, our study demonstrated that the prognosis of CSU is generally favourable in childhood and in most patients with CSU, standard or high-dose antihistamines often resolve symptoms. In patients with longer disease duration at first visit, urticaria that was controlled by a standard dose of antihistamine could predict if the CSU had a good prognosis. This finding may help to increase patient compliance with treatment. Thus, further studies about treatment response and the natural course of CSU in childhood should be performed.
Conflicts of interestNone.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.