Prospective cohort studies have provided useful knowledge about the natural history of asthma. However, most of the studies are conducted in western countries but the course of the disease and long-term outcomes may differ between countries due to environmental and cultural factors.
ObjectiveThe aim of this study is to describe the long-term outcomes of childhood asthma, with data from a follow-up study of at least 10 years, in western Anatolia, Turkey.
MethodsFifty-two patients diagnosed with persistent allergic asthma participated in the study. The patient's demographics, findings on admission, age at onset of disease, time of diagnosis, history of other allergic conditions, history of parental asthma and allergic disorders, presence of pharmacotherapy and immunotherapy were obtained from patients’ records. The factors influencing remission at the end of 10 years follow-up were evaluated.
ResultsA total of 20 patients (38.5%) were on remission at the end of 10 years. The type of allergen, multi-allergen sensitivity, eosinophilia and elevated serum immunoglobulin E on admission, accompanying allergic disorders and atopy in parents, and allergen immunotherapy did not affect the remission rate (p>0.05).
ConclusionChildhood persistent asthma is not a homogeneous clinical entity but high clinical remission rates are obtained in western Anatolia. There is no significant predictor of clinical remission in long term follow-up. Prospective studies should be performed in larger asthmatic populations to obtain further data about the natural course of childhood asthma.
Asthma is a chronic airway inflammation characterised by intermittent, recurrent episodes of wheezing, dry coughing and shortness of breath. It is a major health problem for all ages but childhood asthma represents a special aspect in contrast to adulthood asthma because of the age- and growth-dependent changes in anatomy and physiology of the respiratory system. Paediatricians need to know about the natural history of asthma to answer the questions about whether the disease is life-long or the child will grow up with the disease. A large number of population-based studies have been carried out to investigate the long-term outcomes of the disease but the natural history of childhood asthma is still controversial. The aim of this study is to define the long-term outcomes of allergic asthmatic children over 10 years follow-up and to discuss the factors influencing the natural history of childhood asthma in western Anatolia, Turkey.
Patients and methodsA total of 176 patients diagnosed with persistent allergic asthma approved by allergen skin testing and followed up for at least 10 years in Allergy-Immunology Clinics of Behçet Uz Children's Hospital, a tertiary care hospital for paediatric age, were evaluated for the study. The patients who were not sensitised in the beginning of the follow-up period and the ones diagnosed with mild intermittent asthma were not included in the study. The patients who refused to answer the questions investigating whether they had an attack in the last year were excluded from the study.
Finally, 52 patients participated in the study. All participants agreed to participate according to the principles expressed in the Declaration of Helsinki. The patient's demographics, findings on the time of diagnosis (presence of eosinophilia, increased serum IgE, type of allergen), age at onset of disease, time of diagnosis, history of other allergic conditions, history of parental asthma and allergic disorders, presence of pharmacotherapy (long-term controller medications) and immunotherapy were obtained from patients’ records. Immunotherapy was administered according to WHO and EAACI position papers for allergen immunotherapy.1,2
The presence of >450eosinophils/μL and serum total IgE >100IU/mL were considered eosinophilia and elevated serum IgE, respectively. At the time of the study, patients with no attack, no use of quick-reliever medications in the previous year and no admission to the emergency department for this reason were accepted on clinical asthma remission.
The factors influencing the clinical asthma remission at the end of 10 years follow-up were evaluated. The data were analysed using a statistical software package (SPSS, version 18.0). The Chi-square test or Fisher's exact test, where appropriate, was used to compare proportions in different groups. Factors were considered statistically significant if p values <0.05.
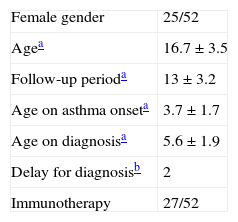
ResultsFifty-two persistent allergic asthmatic patients (age range: 11–26 years; mean age: 16.7±3.5; male/female: 27/25) were followed-up for 13±3.2 (min 10–max 22 years). Mean age on diagnosis was 5.6±1.9 (min 2–max 10 years) and the median time of diagnosis after the onset of symptoms was 2 years (min 0–max 6 years) (Table 1). The frequency of eosinophilia and elevated levels of serum total IgE were 76.9% and 86.5% respectively. The most common aeroallergens in our study group were house-dust mite (61.5%), pollens of grasses-wheat (36.5%), pollens of olive tree (25%), mould (11.5%), animal dander (3.8%) and others (8.5%).
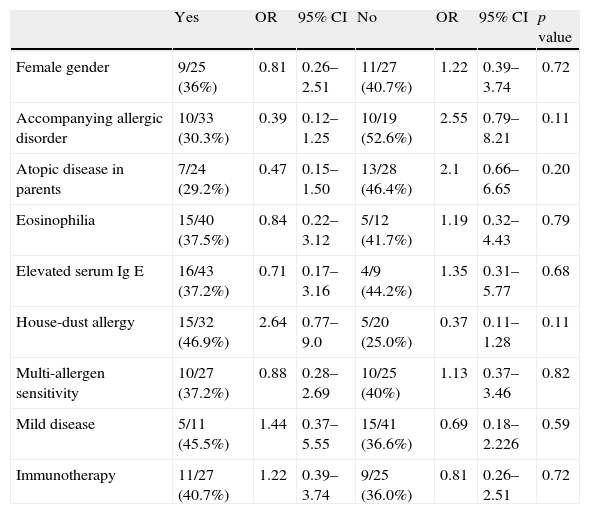
A total of 20 patients (38.5%) were on clinical asthma remission at the end of 10 years. The remission rate in the house-dust mite subgroup was 46.9% and was higher than other allergen's subgroup but the difference was not statistically significant (OR=2.64; 95% CI=0.77–9.0)
The frequency of multi-allergen sensitivity (at least two positive aeroallergen sensitivity on skin-prick test response in a panel of 12 common aeroallergens) was 51.9%. Multi-allergen sensitivity did not affect the remission rate either (OR=0.88; 95% CI=0.28–2.69). Neither eosinophilia and high levels of total Ig E nor the type of aeroallergen was effective on clinical asthma remission.
The frequencies of allergic rhinitis, atopic dermatitis and food allergy were 57.7%, 5.8% and 3.8%, respectively. The remission rate in patients having only asthma was higher (52.6%) than in patients with accompanying allergic disorders (30.3%). However, the difference was not statistically significant (OR=2.55; 95% CI=0.79–8.21).
The frequency of tobacco smoke exposure was 38.5%, in the study group. The remission rate in the smoke-exposure group was 35.5%, while it was 40.6% in the smoke non-exposure group and tobacco smoke exposure seems to be ineffective on the clinical asthma remission.
In our study group, 21.2% of the patients were mild persistent, 76.9% were moderate persistent and 1.9% were severe persistent on follow-up. At the end of 10 years, the rate of remission was 45.5% in the mild persistent asthmatic group, while being 36.6% in the moderate and severe group. The difference was not statistically significant (OR=1.44; 95% CI=0.37–5.55). The remission at the end of 10 years in immunotherapy-administered group and non-administered group was 40.7% and 36.6%, respectively. The effect of immunotherapy on remission could not be documented (OR=1.22; 95% CI=0.39–3.74) (Table 2).
Remission rates of subjects according to risk factors, severity of the disease and immunotherapy.
| Yes | OR | 95% CI | No | OR | 95% CI | p value | |
| Female gender | 9/25 (36%) | 0.81 | 0.26–2.51 | 11/27 (40.7%) | 1.22 | 0.39–3.74 | 0.72 |
| Accompanying allergic disorder | 10/33 (30.3%) | 0.39 | 0.12–1.25 | 10/19 (52.6%) | 2.55 | 0.79–8.21 | 0.11 |
| Atopic disease in parents | 7/24 (29.2%) | 0.47 | 0.15–1.50 | 13/28 (46.4%) | 2.1 | 0.66–6.65 | 0.20 |
| Eosinophilia | 15/40 (37.5%) | 0.84 | 0.22–3.12 | 5/12 (41.7%) | 1.19 | 0.32–4.43 | 0.79 |
| Elevated serum Ig E | 16/43 (37.2%) | 0.71 | 0.17–3.16 | 4/9 (44.2%) | 1.35 | 0.31–5.77 | 0.68 |
| House-dust allergy | 15/32 (46.9%) | 2.64 | 0.77–9.0 | 5/20 (25.0%) | 0.37 | 0.11–1.28 | 0.11 |
| Multi-allergen sensitivity | 10/27 (37.2%) | 0.88 | 0.28–2.69 | 10/25 (40%) | 1.13 | 0.37–3.46 | 0.82 |
| Mild disease | 5/11 (45.5%) | 1.44 | 0.37–5.55 | 15/41 (36.6%) | 0.69 | 0.18–2.226 | 0.59 |
| Immunotherapy | 11/27 (40.7%) | 1.22 | 0.39–3.74 | 9/25 (36.0%) | 0.81 | 0.26–2.51 | 0.72 |
In our study group, all patients were treated with long-term controller medications including ICS (inhaled corticosteroids) (44%), ICS combined with leukotriene modifiers (23%), LABAs (long acting inhale β-agonist) (11.5%), leukotriene modifiers (6%), antihistaminics (7%) and others (9%). Because of the retrospective design of our study, there was not a homogenous treatment regimen on persistent childhood asthma.
DiscussionWe characterised 52 children with persistent allergic asthma over a follow-up period of 10 years. A total of 20 patients (38.5%) were on clinical asthma remission at the end of 10 years. Eosinophilia, high levels of serum IgE, type of allergen, the severity of the disease, immunotherapy or any long-term controller medications seems to be ineffective on clinical remission.
The natural history of asthma can be learned by following cohort studies from birth to adulthood.3 Some cohorts are based on asthma outpatients4,5 while some are based on general population.6,7 Most of the studies are conducted in western countries. However, the course of the disease and long-term outcomes may differ between countries due to environmental and cultural factors.8 Variable remission rates of 14–75% have been reported in unselected population-based or pre-selected cohorts.4–7 This variability could be largely due to a lack of a standard definition for asthma remission and the heterogeneity of the populations from which the estimates were derived. In this current study, we aimed to evaluate the factors influencing clinical remission rates in childhood asthma because it is reported that bronchial hyperreactivity continues also in long-lasting clinical remission in adolescence.9 Clinical asthma remission has been defined as the absence of need for pulmonary medication and does not require any tests (e.g. lung function tests) while the definition of complete asthma remission also includes the absence of bronchial hyperreactivity and normal lung function (FEV1>90% predicted).10,11 This evaluation may have resulted in relatively higher remission rates than in some other reports.
De Marco et al. point out that subjects living in the Mediterranean region have higher incidences and lower remission rates than persons living in the subcontinental region.12 For this reason, many studies must be conducted to evaluate incidence and remission rates in each region. The International Study of Asthma and Allergies in Childhood (ISAAC) reported the asthma prevalence in Turkey as being between 2.8% and 14.5%13,14 and there are only a few reports documenting the factors predicting asthma persistence in childhood from Turkey. Sekerel et al.15 reported diminished airflow, female gender and eosinophilia to predict the disease persistence in early adulthood, from the Middle-Eastern Region of Turkey while Bahçeciler et al.16 reported that respiratory symptoms were significantly predicted by initial FEF 25–75% and sensitivity to allergens and from the Marmara Region, an industrial zone of Turkey. But there is still a lack of data on long-term outcomes and risk factors affecting the persistence of childhood asthma in Turkey and the Aegean Region. This study is conducted in a single-centre clinic, but a reference clinic in a tertiary care hospital for paediatric age, so it serves large number of patients from western Anatolia. Thus, we believe this study respects the outcomes of the entire Aegean Region.
In studies of the natural history of asthma, female gender has been cited as a risk factor for the persistence of symptoms from childhood to adulthood.3,7,15,17,18 Although there are factors associated with airway responsiveness in both males and females, sex-specific factors are reported to contribute to new insights into asthma pathogenesis.18 In this current study, females have a lower remission rate than males but our study is relatively small to document a sex- specific association.
Although allergy and atopy are reported to be risk factors for persistent asthma in childhood,19 this issue is still controversial. Roorda et al.,20 and then Van Beer et al.,21 showed that allergy and atopy do not contribute to the persistence of asthma. In our study, the frequency of eosinophilia and high levels of serum total IgE were 76.9% and 86.5%, respectively. They were not effective on both the severity of the disease and the remission at the end of 10 years. In addition, the remission rate in patients having only asthma was slightly higher (52.6%) than in patients with accompanying allergic disorders (30.3%), but the difference was not statistically significant; so our study could not document an association between atopy, allergy and clinical remission of childhood asthma.
In recent studies, the association of sensitisation to indoor allergens with the development22 and severity23 of asthma in childhood has been recognised. The CAMP study,24 reported that avoiding or limiting exposure to relevant indoor allergens in children who are allergic may alter the course of asthma in childhood. In our study, the most common aeroallergen was house-dust mite allergy (61.5%) and the remission rate in house-dust mite subgroup was 46.9%; higher than other allergen's subgroup in contrast with other reports. This may be associated with the properties of other aeroallergens existing in our region.
Some authors have reported that recent asthma exacerbations contribute strongly and independently to the prediction of a future exacerbation.25 Furthermore, remission is reported to occur mainly in patients with less severe asthma.26 But severity is not a fixed feature of asthma, and may change over months or years, whereas the classification by severity suggests a static feature. Moreover, using severity as an outcome measure has limited value in predicting what treatment will be required and what the response to that treatment might be.27 Indeed, in our study group, the rate of remission was 45.5% in mild persistent asthmatic group, yet was 36.6% in the moderate and severe group. The difference was not statistically significant.
There is convincing evidence that allergen specific immunotherapy induces clinical and immunological tolerance and has long-term efficacy. Subcutaneous immunotherapy has been widely used and has been shown to be effective in reducing symptoms.28 It represents the only treatment modality that might alter the natural course of the disease.29–31 However, it has been reported that immunotherapy for asthma does not provide benefits beyond those achievable with alternative modalities.32 Furthermore, uncommon but severe and nearly fatal systemic reactions and repeated injections leading to serious complaints among patients have restricted the administration.33–36 In our study group, subcutaneous immunotherapy was administered in 51.9% of the patients according to WHO and EAACI position papers for allergen immunotherapy.1,13 The remission at the end of 10 years in immunotherapy-administered group and the non-administered group was 40.7% and 36.6%, respectively. The difference was not statistically significant so our results could not document the efficacy of immunotherapy in childhood asthma. In recent reports, specific immunotherapy is reported to improve asthma related quality of life in childhood.37 In our study, asthma related quality of life or the decrease in the need of quick-reliever medications were not evaluated.
Another argumentative issue on asthma is whether the use of long term controller medications influences the natural history of asthma by reducing inflammation, subsequent remodelling and thus preventing the decline in lung function. Agertoft and Pedersen38 reported the first study to suggest that inhaled corticosteroids influence lung function and they postulated that early intervention may prevent the development of irreversible airway obstruction. START study was a randomised double-blind trial documenting the lesser decline of FEV1 in inhaled budesonide group than placebo while IMPACT study reported no significant difference in post-bronchodilator FEV1 in budesonide, zafirlukast and placebo.39,40 There is not a consistent protocol in our treatment strategies, so we cannot comment on long-term controller medications.
The limitation of our study is its retrospective design. There is insufficient information on patients’ records including data about exclusive breastfeeding rate, number of children and living conditions of the family, socioeconomic status and pet ownership rates that may alter the course of the disease. Moreover, although the lung function tests were not required for the definition of clinical remission, spirometry was performed on patients during clinical follow up. However, the data about lung function tests were not evaluated because of lack of standardisation due to the retrospective design.
ConclusionIn conclusion, this is the first study to report long term outcomes of childhood asthma from western Turkey. As the course of the disease may differ due to environmental factors, the findings may be enlightening for especially Mediterranean physicians documenting high remission rates with no predictor. The limitation of this cross-sectional study is its retrospective design. Prospective studies performed in larger asthmatic populations may obtain further data about the natural course of the disease.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest to declare.
This study has no supporting grants. We are grateful to the staff of Pediatric Allergy Clinics and the children and their parents participating in this study.