The key role of dietary factors in immunotolerance promotion and allergic diseases prevention has been emphasised. The aim of the study was the analysis of the impact of immunomodulatory dietary components, consumed by pregnant women, on the development of cow's milk allergy (CMA) in their offspring.
Materials and methodsFifty-one pairs of mothers and their CMA-offspring were included in the study group. The analysis of a daily intake of selected dietary components was conducted retrospectively with the application of a seven-day diet of a mother in the third trimester of gestation and the authors’ own questionnaire. The Diet 5.D programme was used.
ResultsAn average daily retinol intake by study-group mothers was significantly lower than by control-group mothers and valued 375.6μg/d vs. 543.7μg/d (p=0.040), respectively. Folates intake in the study group was 598.8μg/d vs. 361.1μg/d in the control group (p=0.001). Vitamin D in the study group was statistically lower – 3.6μg/d, comparing to the control group – 6.9μg/d (p=0.038). Average LC-PUFA intake by mothers with allergic children was 0.09g/d, while in the control group 0.18g/d (p=0.016). An analysis of the diet revealed that significantly more mothers of children from the control group (n=12; 48%) consumed fish 2–3 times per month in comparison to the study group (n=9; 17.6%) (p=0.007).
ConclusionsVitamin D, A, LC-PUFA, retinol, riboflavin and fish consumption by pregnant mothers of CMA-children was significantly lower, whereas beta-carotene and folates consumption was significantly higher than that of mothers with non-allergic children.
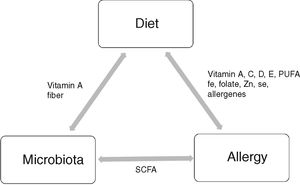
Elimination diet is the most common treatment method of food allergy (FA). However, diet appears to be beneficial not only during the therapy [1]. The key role of dietary factors in the promotion of immunotolerance and prevention of allergic diseases has recently been emphasised [2]. Nutrition and microbiome are the main environmental factors, which together with genetic factors, influence the development of allergic diseases, including cow's milk allergy (CMA). It has been evidenced that a diet determines the quality of microbiota which colonises the gastrointestinal tract [3,4]. It still remains unclear to what extent microbiota contributes to allergy development, but data obtained from animal models strongly suggest a protective role of short chain fatty acids (SCFA), produced during fermentation of fibre and oligosaccharides (propionate, butyrate, acetate) [5]. These metabolites regulate acetylation of Foxp3 and Treg development and thus have an anti-inflammatory effect but also affect epithelial integrity. The SCFA propionate also impacts dendritic cell (DC) biology and the ability to promote T helper 2 (Th2) response. The immune system in turn might affect absorption of nutrients [6].
Nutrition can contribute to the development of allergies during foetal life, after birth, during breastfeeding or bottle feeding, and later after weaning when other foods are introduced. A diet is particularly important in foetal life [1,2,7]. However, it is still unknown if a diet affects the immune system. If it does so, what dietary components, when and in what quantities cause disturbances in the immune system and lead to allergy development? Moreover, not only the quality and quantity of dietary components but also their form may increase the risk of the disease. Tukkola et al. reported an increased risk of developing CMA in children of women taking vitamin D and folate supplements, whereas vitamin D intake from foods during pregnancy was associated with a decreased risk of CMA [8]. Many studies show that maternal diet during pregnancy can influence a subsequent development of allergic outcomes in infants [1,2,9]; however, the results of other studies are contradictory [10].
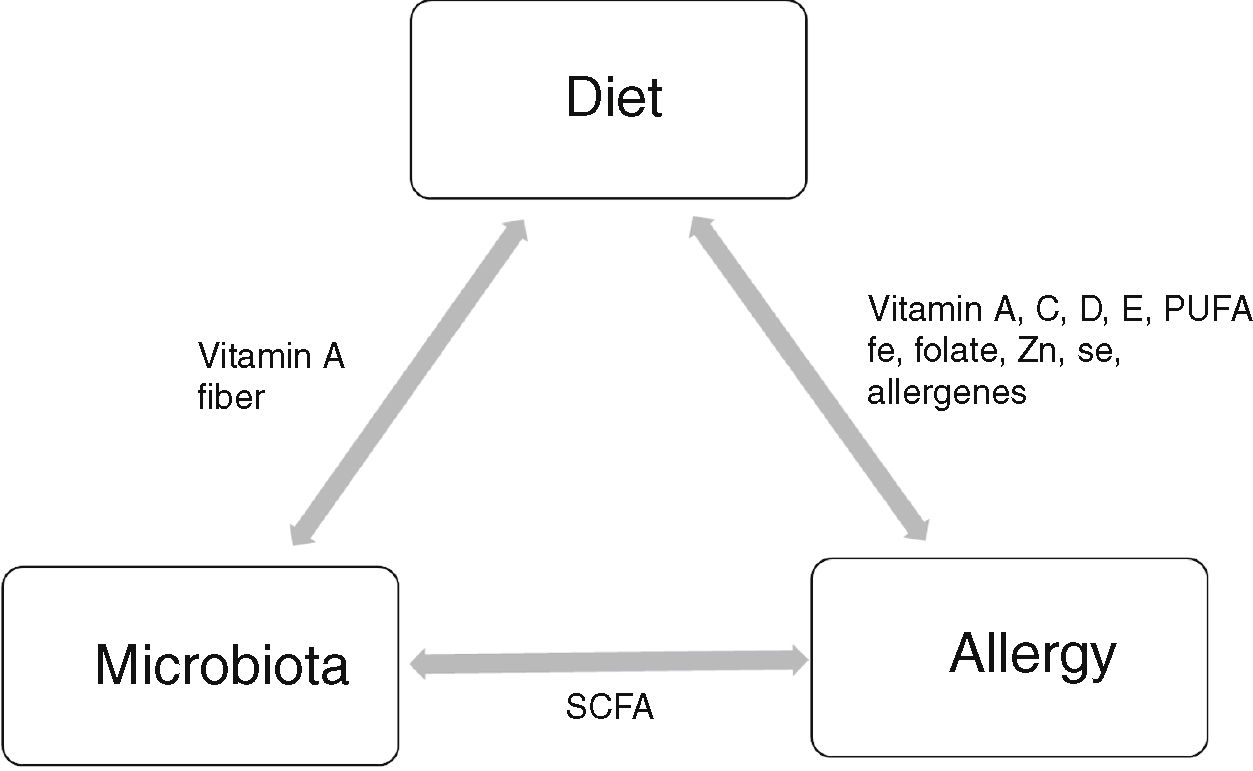
Dietary components have an immunomodulatory effect on the function of the immune system, not only indirectly, through microbiota, but also directly, by increasing cellular activity and enhancing generation of immunoglobulins [11] (Fig. 1). What is important is the fact that dietary components affect the immune system through epigenetic mechanisms, by inducing changes in gene expression and promoting tolerogenic conditions in the intestine [12].
Dietary factors which have immunomodulatory properties include: antioxidants, vitamins, folates, long chain polyunsaturated fatty acids (LC-PUFA), pre- and probiotics [13].
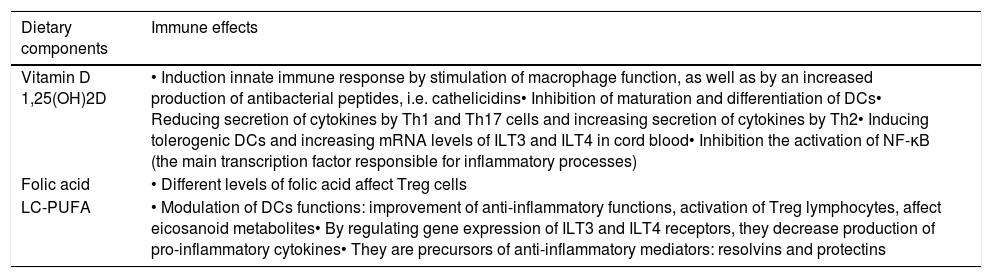
Vitamin A promotes differentiation of monocytes, neutrophils and lymphocytes, including Treg lymphocytes [14], maintains the integrity of mucosal membranes, induces production of TGF-β, as well as is a modulator of gene transcription [15]. Deficiency of vitamin A contributes to decreased subpopulation of CD4 cells [15]. Beta-carotene (β-carotene) stimulates cellular response by enhancing expression of MHC class II cells. Vitamin C appears to modulate systemic and leucocyte-derived cytokines in a complex manner. The effect of vitamin C on cytokine generation appears to depend on the cell type and/or the inflammatory stimulant. Besides, it has a promoting effect on Th1 lymphocytes and an inhibitory effect on Th2 lymphocytes. Vitamin E, found in fats and oils (particularly those which contain LC-PUFA), is responsible for the integrity of cell membranes [16]. Many other dietary components are important in the modulation of the immune system, such as: vitamin D, folic acid and LC-PUFA. Their immunomodulatory functions are presented in Table 1[17–19].
The immunomodulatory effects of dietary components: vitamin D, folic acid, LC-PUFA [17–19].
| Dietary components | Immune effects |
|---|---|
| Vitamin D 1,25(OH)2D | • Induction innate immune response by stimulation of macrophage function, as well as by an increased production of antibacterial peptides, i.e. cathelicidins• Inhibition of maturation and differentiation of DCs• Reducing secretion of cytokines by Th1 and Th17 cells and increasing secretion of cytokines by Th2• Inducing tolerogenic DCs and increasing mRNA levels of ILT3 and ILT4 in cord blood• Inhibition the activation of NF-κB (the main transcription factor responsible for inflammatory processes) |
| Folic acid | • Different levels of folic acid affect Treg cells |
| LC-PUFA | • Modulation of DCs functions: improvement of anti-inflammatory functions, activation of Treg lymphocytes, affect eicosanoid metabolites• By regulating gene expression of ILT3 and ILT4 receptors, they decrease production of pro-inflammatory cytokines• They are precursors of anti-inflammatory mediators: resolvins and protectins |
DCs: dendritic cells; ILT: immunoglobulin-like transcripts; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells.
So far, the relationship between the diet of a pregnant woman and the incidence of allergy in her offspring is still unclear [10]. Therefore, the aim of the study was the analysis of the impact of immunomodulatory dietary components, consumed by pregnant women, on the development of CMA in their children. To the best of our knowledge, this is the first pilot study, conducted in Poland, which shows associations between maternal diet during pregnancy and CMA in infancy.
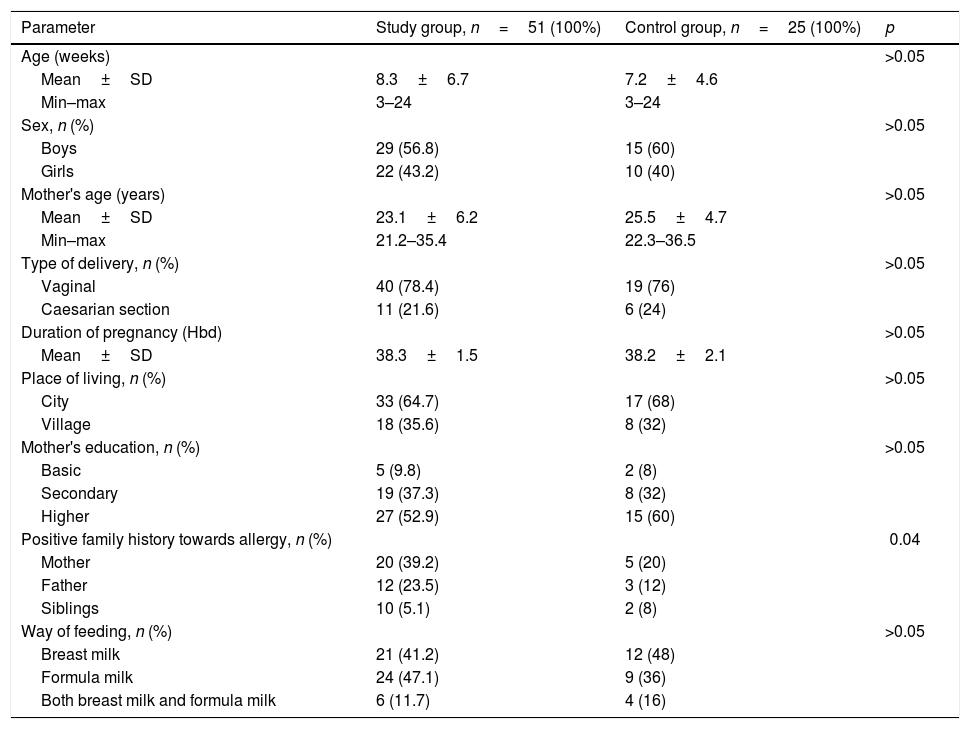
Material and methodsFifty-one pairs consisting of the mother and her child with CMA were included in the study group. They were diagnosed in the Department of Paediatrics, Allergology and Gastroenterology of L. Rydygier Collegium Medicum (CM) in Bydgoszcz, between January 2017 and February 2018. The control group was composed of 25 pairs of mother–child, at a similar age, without allergy. Table 2 presents characteristics of the control and study groups. Inclusion criteria of the study were the following: infants aged 3–24 weeks old, CMA, and a consent given by legal guardians of the babies. Exclusion criteria of the study were: lactose intolerance and other concomitant disorders which might have affected results of a conducted examination. CMA was diagnosed on the basis of anamnesis, physical examination, elimination test and open oral food challenge, according to the guidelines of the European Academy of Allergy and Clinical Immunology, the American Academy of Allergy, Asthma and Immunology and the Polish Society of Allergology [20–22]. In the group of children with CMA, 23 (45.1%) infants had allergic proctocolitis; 23 (45.1%) infants had atopic dermatitis (AD) and 25 (49%) children were affected by functional disturbances of the gastrointestinal tract, such as strong regurgitation, vomiting, colic, constipation, diarrhoea and abdominal bloating. The study group comprised of 41 (80.4%) children with non-IgE-mediated and 10 (19.6%) children with IgE-mediated CMA. All children with IgE-mediated CMA presented with rash/urticaria, three (5.9%) with wheezing and three (5.9%) with angioedema. All these children were sensitised to cow's milk, four (40%) to egg white, three (30%) to hazelnut, three (30%) to soy, two (20%) to peanut, two (20%) to sesame, and one child to wheat.
Characteristics of the study and control groups.
| Parameter | Study group, n=51 (100%) | Control group, n=25 (100%) | p |
|---|---|---|---|
| Age (weeks) | >0.05 | ||
| Mean±SD | 8.3±6.7 | 7.2±4.6 | |
| Min–max | 3–24 | 3–24 | |
| Sex, n (%) | >0.05 | ||
| Boys | 29 (56.8) | 15 (60) | |
| Girls | 22 (43.2) | 10 (40) | |
| Mother's age (years) | >0.05 | ||
| Mean±SD | 23.1±6.2 | 25.5±4.7 | |
| Min–max | 21.2–35.4 | 22.3–36.5 | |
| Type of delivery, n (%) | >0.05 | ||
| Vaginal | 40 (78.4) | 19 (76) | |
| Caesarian section | 11 (21.6) | 6 (24) | |
| Duration of pregnancy (Hbd) | >0.05 | ||
| Mean±SD | 38.3±1.5 | 38.2±2.1 | |
| Place of living, n (%) | >0.05 | ||
| City | 33 (64.7) | 17 (68) | |
| Village | 18 (35.6) | 8 (32) | |
| Mother's education, n (%) | >0.05 | ||
| Basic | 5 (9.8) | 2 (8) | |
| Secondary | 19 (37.3) | 8 (32) | |
| Higher | 27 (52.9) | 15 (60) | |
| Positive family history towards allergy, n (%) | 0.04 | ||
| Mother | 20 (39.2) | 5 (20) | |
| Father | 12 (23.5) | 3 (12) | |
| Siblings | 10 (5.1) | 2 (8) | |
| Way of feeding, n (%) | >0.05 | ||
| Breast milk | 21 (41.2) | 12 (48) | |
| Formula milk | 24 (47.1) | 9 (36) | |
| Both breast milk and formula milk | 6 (11.7) | 4 (16) |
The analysis of a daily intake of selected dietary components was conducted retrospectively with the application of a seven-day diet of a mother in the third trimester of gestation and the authors’ own questionnaire, prepared on the basis of the validated FFQ (food frequency questionnaire) [23].
In order to prepare diets, we have used the Diet 5.D programme (The National Food and Nutrition Institute, 2011). The content of particular dietary components in a diet was determined. Results were compared with the Estimated Average Requirement (EAR) recommended by the National Food and Nutrition Institute [24].
The obtained results were statistically analysed using STATISTICA 13.1 software (Statsoft, Cracov, Poland). Before further analysis, all data were checked for outliers and normality was checked using the Shapiro–Wilk test. Data were presented by descriptive analysis medians (Me), interquartile range (IQR) for non-normal distribution, minimum (Min), maximum (Max), lower quartile (Q1)–upper quartile (Q3). The Mann–Whitney U-test for two independent trials was used to compare quantitative variables in children with and without CMA. The chi-square test and calculated ratios (ORs) with 95% confidence intervals (CIs) were used to compare the distributions of qualitative variables in particular groups. A logistic regression model was applied to identify factors which considerably contribute to CMA. A p-value of <0.05 was considered statistically significant for all analyses.
The study was approved by Ethical committee of CM in Bydgoszcz. Informed consent of the participants was obtained before enrolment. Data confidentiality has been kept.
ResultsAn analysis of children from the study and control groups by age, sex, mode of delivery, duration of gestation, place of residence, maternal age and education and mode of feeding did not reveal significant differences (p>0.05), but allergy in family members was diagnosed significantly more frequently in the study group than in the control group (p=0.04) (Table 2).
A daily energy consumption and intake of selected dietary components in a daily diet of mothers from both groups were determined. No significant differences with regards to energy value of the diet by mothers of children with and without CMA were noted. In mothers of children with allergy, the energy value of the diet was 2652±292.4kcal/d; in the group of children without allergy symptoms, the value was 2537±198.4kcal/d (p>0.05).
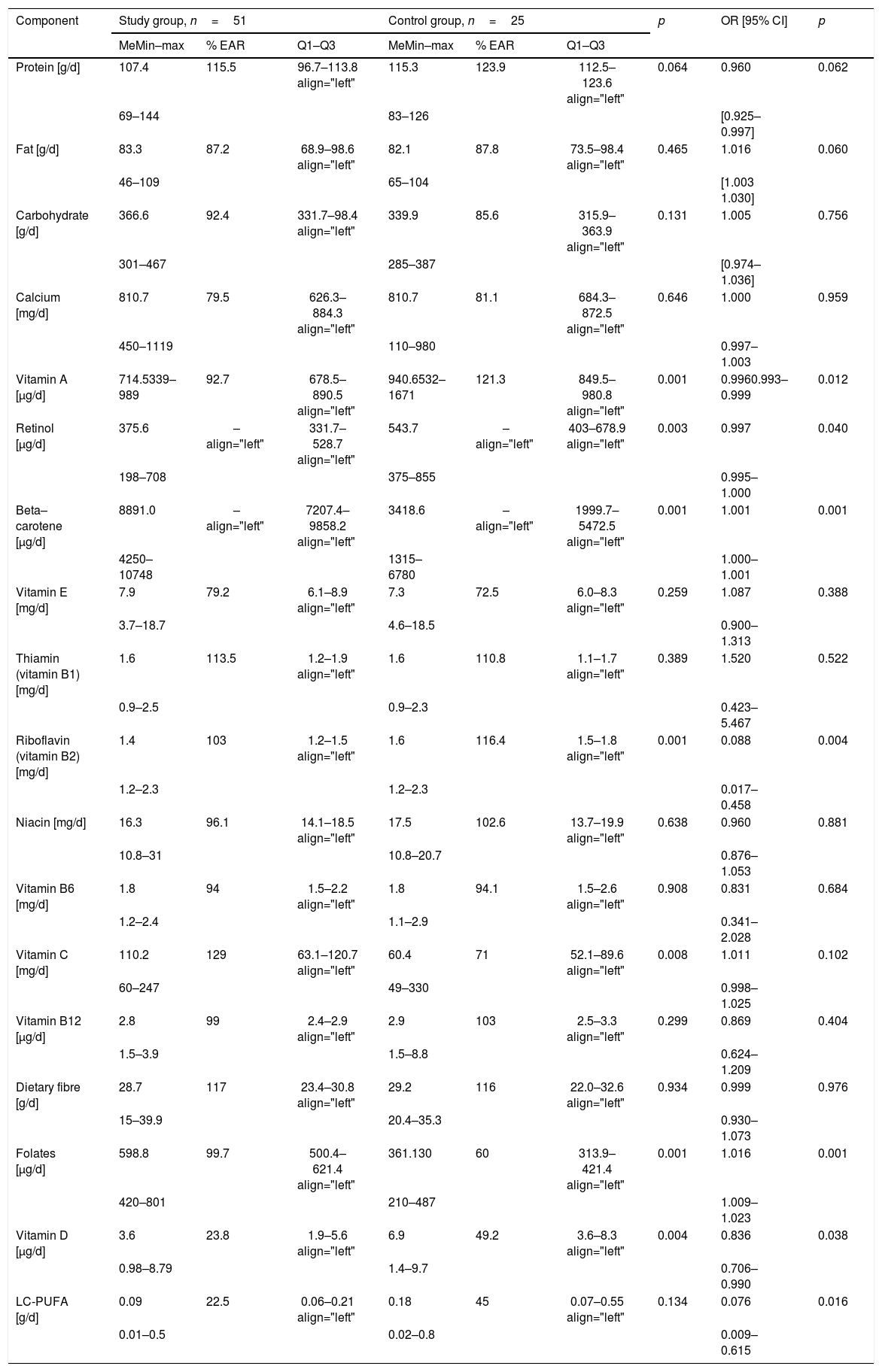
The amount of proteins, fats, carbohydrates intake in the diet of mothers of infants with and without allergy is presented in Table 3. There were no significant differences between them (p>0.05).
Dietary components intake during pregnancy by mothers of offspring with and without CMA, according to the Diet 5 programme and risk of CMA in one factor analysis.
| Component | Study group, n=51 | Control group, n=25 | p | OR [95% CI] | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| MeMin–max | % EAR | Q1–Q3 | MeMin–max | % EAR | Q1–Q3 | ||||
| Protein [g/d] | 107.4 | 115.5 | 96.7–113.8 align="left" | 115.3 | 123.9 | 112.5–123.6 align="left" | 0.064 | 0.960 | 0.062 |
| 69–144 | 83–126 | [0.925–0.997] | |||||||
| Fat [g/d] | 83.3 | 87.2 | 68.9–98.6 align="left" | 82.1 | 87.8 | 73.5–98.4 align="left" | 0.465 | 1.016 | 0.060 |
| 46–109 | 65–104 | [1.003 1.030] | |||||||
| Carbohydrate [g/d] | 366.6 | 92.4 | 331.7–98.4 align="left" | 339.9 | 85.6 | 315.9–363.9 align="left" | 0.131 | 1.005 | 0.756 |
| 301–467 | 285–387 | [0.974–1.036] | |||||||
| Calcium [mg/d] | 810.7 | 79.5 | 626.3–884.3 align="left" | 810.7 | 81.1 | 684.3–872.5 align="left" | 0.646 | 1.000 | 0.959 |
| 450–1119 | 110–980 | 0.997–1.003 | |||||||
| Vitamin A [μg/d] | 714.5339–989 | 92.7 | 678.5–890.5 align="left" | 940.6532–1671 | 121.3 | 849.5–980.8 align="left" | 0.001 | 0.9960.993–0.999 | 0.012 |
| Retinol [μg/d] | 375.6 | – align="left" | 331.7–528.7 align="left" | 543.7 | – align="left" | 403–678.9 align="left" | 0.003 | 0.997 | 0.040 |
| 198–708 | 375–855 | 0.995–1.000 | |||||||
| Beta–carotene [μg/d] | 8891.0 | – align="left" | 7207.4–9858.2 align="left" | 3418.6 | – align="left" | 1999.7–5472.5 align="left" | 0.001 | 1.001 | 0.001 |
| 4250–10748 | 1315–6780 | 1.000–1.001 | |||||||
| Vitamin E [mg/d] | 7.9 | 79.2 | 6.1–8.9 align="left" | 7.3 | 72.5 | 6.0–8.3 align="left" | 0.259 | 1.087 | 0.388 |
| 3.7–18.7 | 4.6–18.5 | 0.900–1.313 | |||||||
| Thiamin (vitamin B1) [mg/d] | 1.6 | 113.5 | 1.2–1.9 align="left" | 1.6 | 110.8 | 1.1–1.7 align="left" | 0.389 | 1.520 | 0.522 |
| 0.9–2.5 | 0.9–2.3 | 0.423–5.467 | |||||||
| Riboflavin (vitamin B2) [mg/d] | 1.4 | 103 | 1.2–1.5 align="left" | 1.6 | 116.4 | 1.5–1.8 align="left" | 0.001 | 0.088 | 0.004 |
| 1.2–2.3 | 1.2–2.3 | 0.017–0.458 | |||||||
| Niacin [mg/d] | 16.3 | 96.1 | 14.1–18.5 align="left" | 17.5 | 102.6 | 13.7–19.9 align="left" | 0.638 | 0.960 | 0.881 |
| 10.8–31 | 10.8–20.7 | 0.876–1.053 | |||||||
| Vitamin B6 [mg/d] | 1.8 | 94 | 1.5–2.2 align="left" | 1.8 | 94.1 | 1.5–2.6 align="left" | 0.908 | 0.831 | 0.684 |
| 1.2–2.4 | 1.1–2.9 | 0.341–2.028 | |||||||
| Vitamin C [mg/d] | 110.2 | 129 | 63.1–120.7 align="left" | 60.4 | 71 | 52.1–89.6 align="left" | 0.008 | 1.011 | 0.102 |
| 60–247 | 49–330 | 0.998–1.025 | |||||||
| Vitamin B12 [μg/d] | 2.8 | 99 | 2.4–2.9 align="left" | 2.9 | 103 | 2.5–3.3 align="left" | 0.299 | 0.869 | 0.404 |
| 1.5–3.9 | 1.5–8.8 | 0.624–1.209 | |||||||
| Dietary fibre [g/d] | 28.7 | 117 | 23.4–30.8 align="left" | 29.2 | 116 | 22.0–32.6 align="left" | 0.934 | 0.999 | 0.976 |
| 15–39.9 | 20.4–35.3 | 0.930–1.073 | |||||||
| Folates [μg/d] | 598.8 | 99.7 | 500.4–621.4 align="left" | 361.130 | 60 | 313.9–421.4 align="left" | 0.001 | 1.016 | 0.001 |
| 420–801 | 210–487 | 1.009–1.023 | |||||||
| Vitamin D [μg/d] | 3.6 | 23.8 | 1.9–5.6 align="left" | 6.9 | 49.2 | 3.6–8.3 align="left" | 0.004 | 0.836 | 0.038 |
| 0.98–8.79 | 1.4–9.7 | 0.706–0.990 | |||||||
| LC-PUFA [g/d] | 0.09 | 22.5 | 0.06–0.21 align="left" | 0.18 | 45 | 0.07–0.55 align="left" | 0.134 | 0.076 | 0.016 |
| 0.01–0.5 | 0.02–0.8 | 0.009–0.615 | |||||||
Me: median; EAR: estimated average requirement; Q1: lower quartile; Q3: upper quartile; OR: odd ratio; CI: confidence interval.
Average intake of calcium, retinol, beta-carotene, vitamins: A, E, B1, B2, B6, B12, C and vitamin D, niacin, folate, LC-PUFA as well as dietary fibre – their median, range min–max, % of EAR, values of Q1–Q3 as well as OR, 95% CI are shown in Table 3. Significant differences in the intake of vitamin A, retinol, beta-carotene, riboflavin, vitamin C, folates and vitamin D have been shown. Mothers of children with CMA consumed more folates, beta-carotene and vitamin C during pregnancy, while less vitamin A, retinol, riboflavin and vitamin D. No statistical significance was found between the groups regarding vitamin E, thiamine, niacin, vitamin B6, B12, LC-PUFA and dietary fibre intake (Table 3).
Risk factors in multiple logistic regression analyses: in order to identify the key factors from maternal diet during the third trimester of pregnancy contributing to allergy in infancy a logistic regression model was applied. A number of potential immunomodulatory risk factors for allergy were tested in univariate models. No significant associations were found with calcium, vitamin B6, C, B12, E, thiamine, niacin and fibre. Significant associations with allergic diseases in infancy were found for the following parameters: vitamin A, retinol, beta-carotene, riboflavin, folates, vitamin D and LC-PUFA (Table 3). Factors with significant association in the univariate analyses were considered in multivariate analysis. The analysis revealed that higher intake of LC-PUFA reduced almost 100 times the risk of allergy in children (aOR=0.027; 95%CI 0.001–0.735; p=0.032), while vitamin A by 1% (aOR=0.996; 95%CI 0.993–1.000; p=0.042). The positive family history towards allergy increased the risk of CMA in infancy almost six-fold (aOR=6.224; 95%CI 1.213–31.935; p=0.028), independently of the diet components.
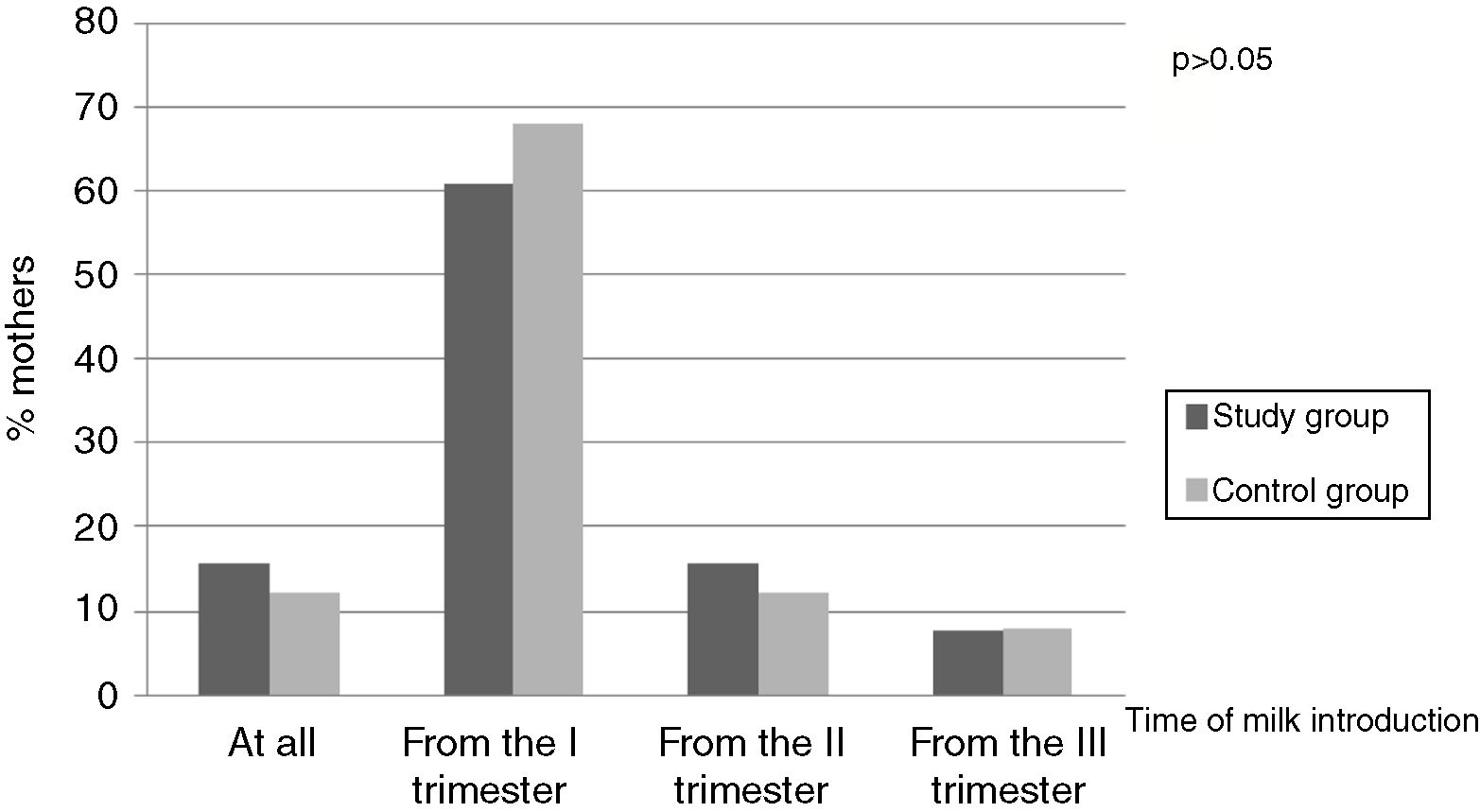
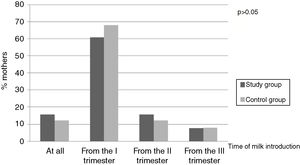
On the basis of the questionnaire the time of introduction of milk and dairy products in the study groups was determined. The majority of women started the consumption of the above alimentary products as early as in the first trimester of pregnancy – 31 (60.8%) mothers of children with CMA and 17 (68%) mothers of healthy children (p>0.05) (Fig. 2).
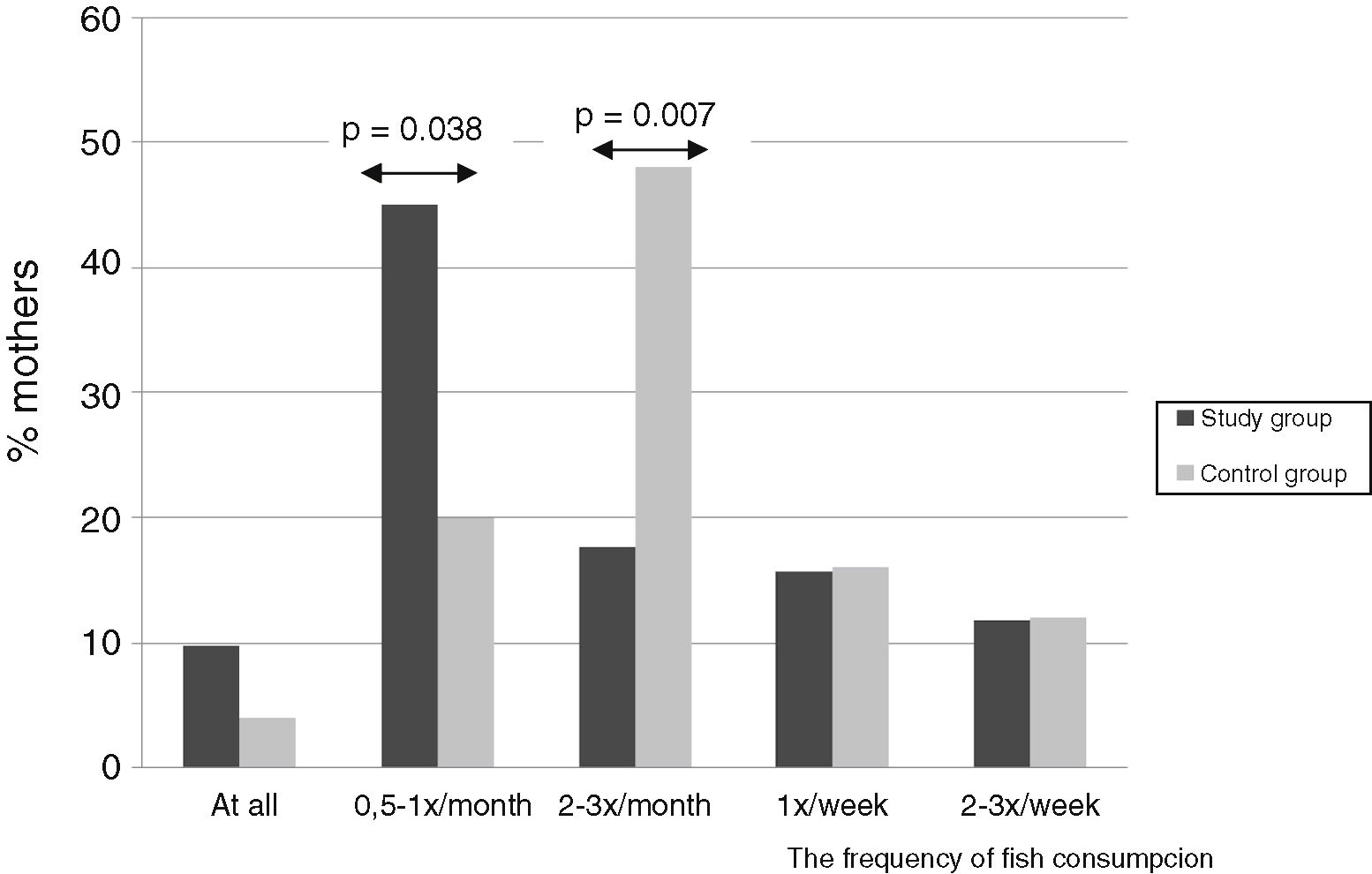
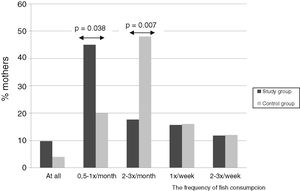
Mothers of children without allergy significantly more frequently consumed fish in comparison to mothers of allergic children. An analysis of the diet revealed that significantly more mothers of children from the control group (n=12; 48%) consumed fish 2–3 times per month in comparison to the study group (n=9; 17.6%) (p=0.007) (Fig. 3). Multivariate analysis showed that the consumption of fish 2–3 times/week reduced the risk of CMA 10 times (aOR=0.164; 95%CI 0.045–0.605; p=0.007).
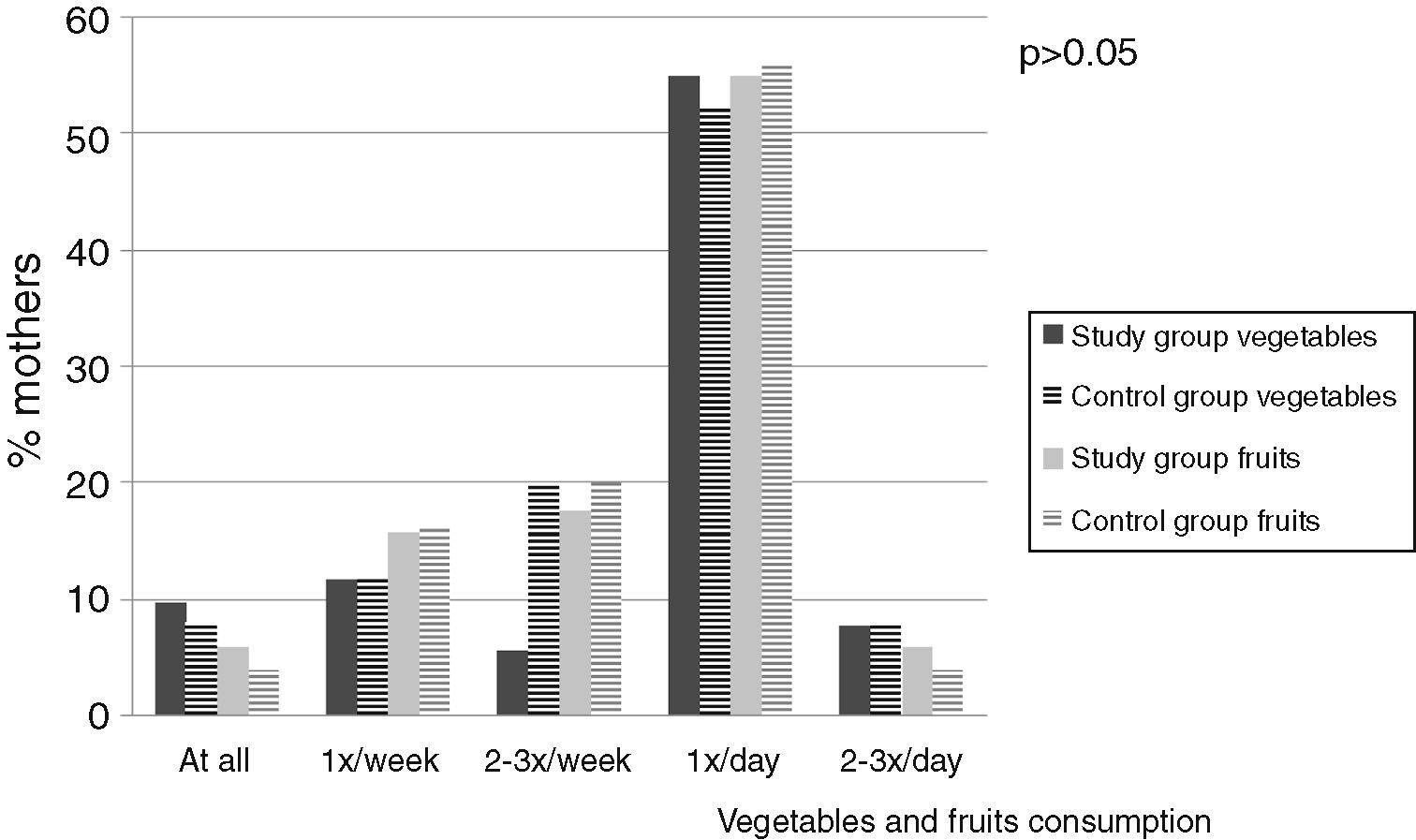
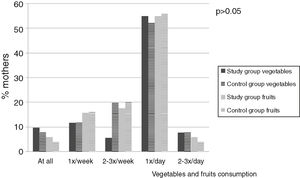
Fig. 4 presents an analysis of vegetable and fruit consumption. In both the groups, vegetables and fruit were usually consumed once a day. Mothers of allergic children equally often ate vegetables and fruit (28 women, i.e. 54.9%). With regards to the control group, 13 (53%) women ate vegetables once a day and 14 (56%) mothers consumed fruit once a day (p>0.05) (Fig. 4).
DiscussionMaternal diet during pregnancy might be one of the factors that influences foetal immune responses associated with childhood allergy [1,2]. Our study revealed a statistically lower intake of vitamin A, B2, D, LC-PUFA, retinol and a higher intake of beta-carotene and folates during pregnancy by mothers of CMA children in comparison to mothers of children without allergy. LC-PUFA and vitamin A consumption by women during the third trimester of pregnancy were independent risk factors for CMA in infancy, adjusted for the family history towards allergy.
Allan et al. imply that a lower intake of vitamin E in pregnancy was associated with a higher risk of asthma in children before the age of 10 years [25]. According to Maslova et al., a higher intake of vitamins E and A protects against allergic rhinitis (AR) [26], and a diet rich in vitamin C, applied by pregnant women, reduces the risk of wheezing in their babies [27]. According to Nurmatov et al., mothers’ diet rich in vitamin A affects the lower prevalence of atopic diseases in children [16]. The results of our study did not show differences in the groups with regard to the consumption of vitamins E and C but mothers from the study group consumed significantly less vitamin A compared to the control group.
The role of vitamin D as a risk factor of allergic diseases is still unclear. Our study revealed a significantly lower intake of vitamin D in mothers of children with CMA in comparison to that observed in the control group, which might imply a relationship with allergy development. The results concur with results obtained by Allen et al., who proved that deficiency of vitamin D in pregnancy significantly increased the risk of sensitisation and occurrence of FA in children, aged 12 months [28] as well as being associated with an increased risk of asthma development in the first ten years of life [25]. However, results of studies on the relationship between vitamin D and the development of allergy are conflicting. According to Miyoka et al., a higher intake of vitamin D in pregnancy increased the risk of AD in their children [29].
Our study confirmed a significantly lower intake of retinol by women whose babies demonstrated allergy symptoms. A similar observance was made by Oh et al. They revealed that a higher intake of retinol decreases the risk of AD [30]. The authors of the study noted significantly higher consumption of beta-carotene in mothers of children with allergy, in contrast to Miyake et al., who observed that a higher intake of this dietary component by pregnant women might protect against a development of AD in their children [14].
The amount of dietary fibres consumed by mothers of children with and without allergy was not different. Fibre has important anti-inflammatory properties, particularly due to the fact that it can generate SCFAs, which stimulate the production of Treg lymphocytes, essential for the development of immunotolerance [31].
Our study revealed that mothers of children with CMA consumed much more folates. Folate, a known methyl donor impacting methylation status, when administered at specific doses, causes a reverse effect, due to the epigenetic mechanism. Specifically, high doses of folic acid (≥5mg/day) in late pregnancy are an established risk factor for allergy [10]. Tuokkola et al. claim that consumption of folic acid during pregnancy is associated with an increased risk of CMA [8]. McStay et al. by basing on a systematic review of studies, noted that folate intake may increase the risk of allergy in children [32].
Fat in the diet of pregnant woman is important mainly in the context of fatty acid composition. Omega-3 fatty acids modulate inflammation by affecting Toll-like receptors (TLRs), related to adequate response to bacteria and other microorganisms [33]. There is a speculation that n−3 LC-PUFA may prevent allergy development through inhibition of inflammation. Eight trials, involving 3366 women and their 3175 offspring, were included in the review. In these trials, women were supplemented with n−3 LC-PUFA during pregnancy, or both pregnancy and lactation. Although there was a clear reduction in children's sensitisation to egg and sensitisation to any allergen between 12 and 36 months of age, when mothers were supplemented with n−3 LC-PUFA, the authors concluded that there is limited evidence to support maternal n−3 LC-PUFA supplementation during pregnancy and/or lactation in order to reduce allergic disease in children [33]. In our study, mothers of children diagnosed with CMA demonstrated a significantly lower intake of LC-PUFA. Palmer et al. confirmed that children of 707 mothers who consumed greater amounts of LC-PUFA in pregnancy, were less frequently diagnosed with sensitisation to eggs and AD, in comparison to children of mothers consuming smaller amounts of LC-PUFA [34]. Turner et al. showed that fish oil reduces sensitisation to egg and to peanut but opposed to sensitisation, there is no evidence of the impact on food allergy outcomes [35]. The results of other studies did not confirm the preventive role of maternal n−3 LCPUFA supplementation in allergy development [33,36].
We have also observed a relationship between consumption of fish and risk of childhood allergy. Children of mothers who included fish in their diet in pregnancy were diagnosed with CMA less frequently. A similar observation was made by Sousenthaler et al. They revealed that a high fish intake in the last four weeks of pregnancy was reversely correlated with the incidence of AD in children below the age of two years [37].
Fruit and vegetables are important factors with immunomodulatory properties and play a role in the prevention of allergy. It was confirmed that consumption of fruit and vegetables by pregnant women decreases the risk of asthma [29]. Alvarez Zallo et al. observed that high fruit consumption during the pregnancy has a protective effect against “wheezing” in 12-month-old infants [38]. We did not note differences regarding the level of fruit and vegetable consumption between the groups. Similarly Loo et al. did not find any associations between the maternal “vegetables and fruit” dietary pattern and allergic outcomes: sensitisation, AD, AR, wheezing in children after 18 months as well as after 36 months [9]. According to Nurmatov, there is very little epidemiological evidence that nutrients and alimentary products have a protective effect on the prevention of asthma and allergic diseases. However, such a relationship between the prevention of the above diseases and vitamins A, D, E, Zn, fruit and vegetables seems to be strong. Alimentary products included in the Mediterranean diet are particularly beneficial in asthma prevention [16].
The presented study does not demonstrate differences with regards to time of including milk in the diet of mothers of children with and without allergy. According to Katz et al., early exposure to milk allergens protects against allergy in the future [39]. Tuokolla et al. showed that high maternal consumption of dairy products during pregnancy may protect children from developing CMA, suggesting antigen-specific induction of tolerance in the pre-natal period [40]. In contrast to Tuokolla, Järvinen discovered that maternal peanut exposure during pregnancy has no impact on the development of peanut allergy in their children [41].
There are some limitations of our study: retrospective design, small group, the lack of uniformity of the study group and the diet evaluation only from the third trimester. Due to a retrospective design of that study, taking into account that the shorter period between CMA confirmation in a child and third trimester of pregnancy, the accuracy of diet data obtained from the mothers will be better. Therefore, we chose the third trimester for diet data collection. The strengths of our study are that it is population-based, including young children form the risk group and without a risk, the analysis of diet using the Diet 5 programme and doctor diagnosed CMA.
The systematic review and meta-analysis, including 26 studies on maternal diet and future risk of allergy, published by Garcia-Larsen et al., supports a relationship between maternal diet and risk of immune-mediated diseases in the child. Maternal probiotic and fish oil supplementation may reduce the risk of eczema and allergic sensitisation to food, respectively [1]. New trends in the therapy of allergic diseases in the future will probably involve the manipulation of microbiota and diet. Identification of components of the maternal diet as early as in the gestation period could be helpful in the implementation of primary prophylaxis.
ConclusionsMaternal diet during pregnancy can impact on the subsequent development of allergic outcomes in the offspring. LC-PUFA and vitamin A consumption by women during the third trimester of pregnancy are independent risk factors for CMA in infancy. The analysis of a diet with the use of the Diet 5 programme allows for a more detailed evaluation of the role of particular components in the diet of pregnant women than an analysis of a diet made on the basis of the questionnaire only. The relationship between the diet of pregnant women and the incidence of food allergy in their children requires further research.
FundingMK, IS, EŁ-R and AK declare that they have no significant competing financial, professional or personal interests that might have influenced the performance or presentation of the work described in this manuscript.
This manuscript received no funding.
Author's contributionMK, EŁ-R collected data, carried out the initial analyses, drafted the initial manuscript.
IS collected data, carried out the initial analyses and made a substantial contribution to the analysis and interpretation of data.
AK conceptualised and designed the study and critically reviewed the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of interestsThe authors have no conflict of interest to declare.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects.
Protection of human subjects in researchThe authors declare that no experiments were performed on humans for this investigation.