The natural history of progression from acute urticaria (AU) to chronic urticaria (CU) remains poorly understood. This study aimed to investigate the potential triggers of AU attacks and factors associated with their duration, as well as the factors which may be predictive of progression to CU.
MethodsThe study included 281 AU patients (AU group). Data were obtained from 207 AU patients retrospectively and from 74 AU patients prospectively. The CU group consisted of 953 patients, whose data were previously published.
ResultsAccording to the medical history, the most common potential triggers of AU attacks were drugs (38.1%); infections (35.2%); stress (24.7%); and foods (17.8%). Attack duration was shorter in cases in which food (p=0.04) or infection (p=0.04) was the suspected trigger. Patients with a history of rhinitis (p=0.04) and food allergy (p=0.04), and positive skin prick test results for pollens (p=0.02) and dog (p=0.02) also had attacks of shorter duration. Patients with asthma had attacks of longer duration (p=0.01). Based on history and/or provocation test results, the prevalence of non-steroidal anti-inflammatory drug hypersensitivity (NSAIDH) was significantly higher in the CU group than the AU group (24.9% vs. 4.3%, respectively, (p<0.01)), as was antibiotic hypersensitivity (10.6% vs. 4.6%, respectively, (p<0.01)) and food allergy (18.3% vs. 3.9%, respectively, (p<0.01)). NSAIDH (OR: 7.97; 95%CI: 4.33–14.66; p<0.01) and food allergy (OR: 5.17; 95%CI: 2.71–9.85; p<0.01) were observed to be independent factors associated with CU.
ConclusionsAs NSAIDH and food allergy were associated with CU, their presence should be carefully evaluated in patients with AU in order to predict progression to CU.
Urticaria is characterised by the sudden appearance of wheals and/or angio-oedema, and affects as much as 20% of the general population at least once during their lifetime. According to the duration of symptoms, urticaria is classified as acute (duration<6 weeks) or chronic (duration>6 weeks)1; however, as many as 25% of patients that present as acute urticaria (AU) or angio-oedema of unknown cause may eventually be diagnosed as chronic urticaria (CU).2
Infections, drugs, and foods are the most common triggers of attacks of AU, but in >50% of patients the condition remains idiopathic.3–5 A detailed history and physical examination are necessary for the evaluation of AU patients. Extensive laboratory investigation and allergy testing are not indicated, except in severe cases of AU in which a type 1 allergy is the suspected cause. On the other hand, CU requires a more comprehensive diagnostic workup to ensure adequate control of the disease and to prevent subsequent attacks.1
The natural history of the progression from AU to CU remains poorly understood. As such, the present study aimed to identify the potential triggers of AU attacks and the factors associated with their duration, as well as the factors which may be predictive of progression to CU.
Materials and methodsThe study included 281 AU patients (AU group) and 953 CU patients (CU group). Data were collected from 207 AU patients retrospectively (retrospective data subgroup) and from 74 AU patients prospectively (prospective data subgroup). We have been collecting the short clinical records of every patient in our clinic since January 1991. First, all AU patients seen between January 1991 and June 2010 were identified, and then their detailed medical records were reviewed. Only AU patients that were symptom free after six weeks of follow-up were included in the study. Data on age, gender, previous history of urticaria and/or angio-oedema, history of infections, suspicious medication or food intake, exposure to contact materials, psychological stress, and insect bites, personal and family history of atopic diseases (asthma, rhinitis, drug allergy, food allergy, metal allergy, bee venom allergy), accompanying diseases and drug use, clinical presentation (distribution of wheals and the presence of angio-oedema), and medications used to control attacks were collected. The results of laboratory tests, including complete blood count, biochemistry, urinalysis, and stool examination for parasites, and radiological examinations were also recorded, if performed. These laboratory tests were not routinely performed for the evaluation of AU patients, but performed in cases in which infectious and/or systemic diseases were suspected.
In the retrospective data subgroup, skin prick test (SPT) and provocation test results were also retrieved from the medical records, if performed. In routine clinical practice we perform SPTs in all AU patients with a history of atopic disease(s) or in those in whom a type 1 allergic reaction is suspected as a trigger for attacks of AU. SPTs were performed during follow-up visits after all symptoms resolved and cessation of antihistamines. The SPT panel included common aeroallergens (Dermatophagoides pteronyssinus, Phleum pratense, Olea europea, Artemisia vulgaris, Parietaria officinalis, Corylus avellana, Betula verrucosa, cat, dog, Alternaria alternata, Cladosporium herbarum, Aspergillus mixture, and Blatella germanica) and foods (corn, hazelnut, peanut, walnut, chicken, egg, lamb, milk, orange, lemon, banana, peach, cherry, cress, radish, celery, onion, tomato, bean, soybean, pea, carrot, mackerel, crab, shrimp, and mussel), which was obtained from ALK (Denmark), Greer (USA), or Alyostal (France) and changed from time to time during the 20-year period. The tests were performed according to published guidelines.6 Histamine and saline were used as positive and negative controls, respectively. A positive reaction was defined as a wheal with a geometric mean diameter ≥3mm. Serum-specific IgE levels in response to aeroallergens and food allergens were measured using a Pharmacia CAP system (Pharmacia Diagnostic AB, Uppsala, Sweden) when SPT could not be performed. Drug provocation tests were performed to confirm the diagnosis of drug hypersensitivity or to identify safe alternative drugs 4–6 weeks after an attack of AU.
The prospective data subgroup included 74 AU patients that presented directly to our clinic or via referral from the adult emergency department of our hospital between July 2010 and January 2011. As in the retrospective data subgroup, demographic data and clinical presentation of the patients were recorded, and the potential triggers of attacks of AU were investigated. Medical treatment was initiated according to published guidelines7 and all patients were followed-up by the same physician until remission. The study protocol was approved by the Hacettepe University Institutional Review Board and written informed consent was obtained from all patients in the prospective subgroup.
The CU group included 953 patients that presented to our clinic between January 1991 and June 2006. Data for these 953 patients were previously published.8
Data are expressed as frequency for categorical variables, and as median (interquartile range) or mean±SD for continuous variables, where appropriate. Between-group comparisons were made using the chi-square test for categorical values and Student's t-test for continuous variables, where appropriate. Fisher's exact test was used to compare categorical variables when ≥25% of the expected cell counts was <5. The Mann–Whitney U test was used to compare continuous variables that were not distributed normally. Logistic regression analysis was used to assess the independent association between the factors that differed significantly in the AU and CU groups based on univariate analysis. Two-sided p values <0.05 were considered statistically significant in all comparisons. SPSS v.15.0 was used for all statistical analyses.
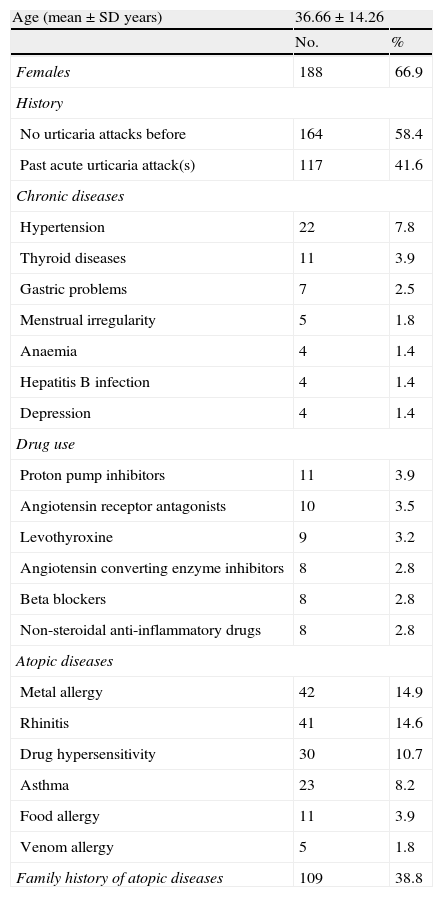
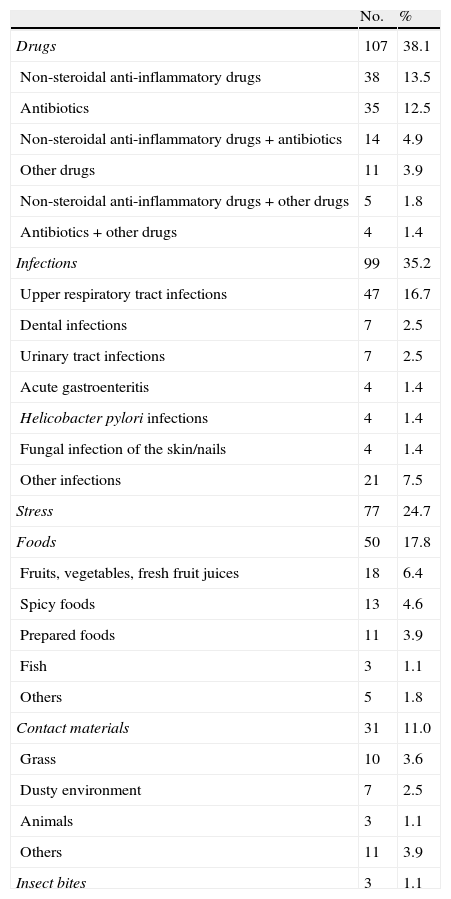
ResultsIn total, the study included 281 AU patients (207 in the retrospective data subgroup and 74 in the prospective data subgroup) with a mean age of 36.66±14.26 years (range: 15–81 years). In all, 238 (84.7%) patients presented with generalised urticarial wheals. Angio-oedema accompanied urticaria in 101 (35.9%) patients (Table 1). The most common history-based potential triggers of attacks of AU were drugs (38.1%), followed by infections (35.2%), stress (24.7%), and foods (17.8%). Among the 107 patients with possible drug-related attacks of AU, 38 used non-steroidal anti-inflammatory drugs (NSAIDs) only, and 35 used antibiotics only. Beta-lactams were the most commonly used (41.5%) antibiotics. The most common infections were upper respiratory tract infections (n=47). Laboratory and/or radiological investigations showed that some of the patients had urinary tract infections (n=7), pneumonia (n=1), empyema (n=1), acute hepatitis (n=1), and parasitic infection (Dientamoeba fragilis) (n=1) (Table 2). In all, 60 (21.4%) patients did not report any potential trigger and 136 (48.4%) had >1 trigger.
Demographical and clinical characteristics of the 281 acute urticaria patients.
| Age (mean±SD years) | 36.66±14.26 | |
| No. | % | |
| Females | 188 | 66.9 |
| History | ||
| No urticaria attacks before | 164 | 58.4 |
| Past acute urticaria attack(s) | 117 | 41.6 |
| Chronic diseases | ||
| Hypertension | 22 | 7.8 |
| Thyroid diseases | 11 | 3.9 |
| Gastric problems | 7 | 2.5 |
| Menstrual irregularity | 5 | 1.8 |
| Anaemia | 4 | 1.4 |
| Hepatitis B infection | 4 | 1.4 |
| Depression | 4 | 1.4 |
| Drug use | ||
| Proton pump inhibitors | 11 | 3.9 |
| Angiotensin receptor antagonists | 10 | 3.5 |
| Levothyroxine | 9 | 3.2 |
| Angiotensin converting enzyme inhibitors | 8 | 2.8 |
| Beta blockers | 8 | 2.8 |
| Non-steroidal anti-inflammatory drugs | 8 | 2.8 |
| Atopic diseases | ||
| Metal allergy | 42 | 14.9 |
| Rhinitis | 41 | 14.6 |
| Drug hypersensitivity | 30 | 10.7 |
| Asthma | 23 | 8.2 |
| Food allergy | 11 | 3.9 |
| Venom allergy | 5 | 1.8 |
| Family history of atopic diseases | 109 | 38.8 |
Potential triggers of the acute urticaria attacks.
| No. | % | |
| Drugs | 107 | 38.1 |
| Non-steroidal anti-inflammatory drugs | 38 | 13.5 |
| Antibiotics | 35 | 12.5 |
| Non-steroidal anti-inflammatory drugs+antibiotics | 14 | 4.9 |
| Other drugs | 11 | 3.9 |
| Non-steroidal anti-inflammatory drugs+other drugs | 5 | 1.8 |
| Antibiotics+other drugs | 4 | 1.4 |
| Infections | 99 | 35.2 |
| Upper respiratory tract infections | 47 | 16.7 |
| Dental infections | 7 | 2.5 |
| Urinary tract infections | 7 | 2.5 |
| Acute gastroenteritis | 4 | 1.4 |
| Helicobacter pylori infections | 4 | 1.4 |
| Fungal infection of the skin/nails | 4 | 1.4 |
| Other infections | 21 | 7.5 |
| Stress | 77 | 24.7 |
| Foods | 50 | 17.8 |
| Fruits, vegetables, fresh fruit juices | 18 | 6.4 |
| Spicy foods | 13 | 4.6 |
| Prepared foods | 11 | 3.9 |
| Fish | 3 | 1.1 |
| Others | 5 | 1.8 |
| Contact materials | 31 | 11.0 |
| Grass | 10 | 3.6 |
| Dusty environment | 7 | 2.5 |
| Animals | 3 | 1.1 |
| Others | 11 | 3.9 |
| Insect bites | 3 | 1.1 |
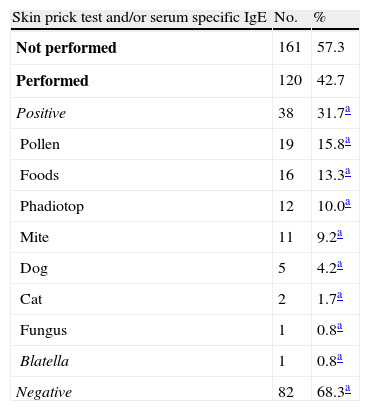
SPTs and/or serum-specific IgE measurements were performed in 120 (42.7%) of all AU patients and 38 had positive results (Table 3). Drug provocation tests were performed in 23 (21.5%) of the 107 AU patients with a suspicious drug intake history, of which 15 were performed to confirm drug allergy -but none were positive. In 12 out of these 15 patients there were accompanying infections (upper respiratory tract infections (n=10), lower respiratory tract infection (n=1), and Helicobacter pylori infection (n=1)). Provocation tests were performed to identify safe alternative drugs in eight AU patients, of which one with a history of ibuprofen use had a positive result and developed urticarial wheals in response to the meloxicam challenge test.
Results of allergy tests in acute urticaria patients.
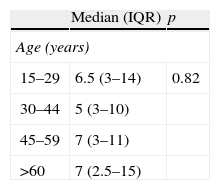
In all, 161 (57.4%) AU patients were treated with systemic steroids and antihistamines. In 103 (36.6%) of the patients only antihistamines were used; the remaining 17 (6.0%) patients did not receive any treatment, because their attack of AU resolved by the time of presentation. Attack duration was known in 265 (94.3%) of the AU patients. Mean±SD duration of the attacks of AU was 9.55±9.64 d (range: 1–40d). The association between patient and attack characteristics, and the duration of attacks was investigated. The attacks tended to have shorter durations when there were foods (p=0.04) or infections (p=0.04) as the potential triggers. Patients with a history of rhinitis (p=0.04), allergic reactions to foods (p=0.04), and pollen and dog sensitivity based on SPT (p=0.02 and p=0.02, respectively) also had shorter attack durations. Patients with asthma had attacks of longer duration (p=0.01) (Table 4).
Analysis of the factors that may influence the duration of the acute urticaria attacks.
| Median (IQR) | p | |
| Age (years) | ||
| 15–29 | 6.5 (3–14) | 0.82 |
| 30–44 | 5 (3–10) | |
| 45–59 | 7 (3–11) | |
| >60 | 7 (2.5–15) | |
| Yes | No | p | |
| Median day(s) (IQR) | Median day(s) (IQR) | ||
| Female gender | 7 (3–10) | 5 (3–15) | 0.98 |
| ACE inhibitor use | 10 (4.5–22.5) | 6 (3–12) | 0.29 |
| Beta blocker use | 7 (5–10) | 7 (3–14) | 0.69 |
| Atopic disease | 6 (2–11) | 7 (3–14) | 0.49 |
| Asthma | 10 (7–30) | 5 (2–10) | 0.01 |
| Rhinitis | 4 (1–10) | 7 (3–14.5) | 0.04 |
| Food allergy | 1 (1–4) | 7 (3–14) | 0.04 |
| Drug hypersensitivity | 7 (2.5–16) | 7 (3–10) | 0.46 |
| Metal allergy | 7 (2–12) | 5.5 (3–14) | 0.72 |
| Venom allergy | 17.5 (8–25) | 7 (3–12) | 0.25 |
| Familial atopy | 5 (2–13) | 7 (3–14) | 0.61 |
| Possible aetiological factors | |||
| Drugs | 5 (2–10) | 7 (3–15) | 0.15 |
| Foods | 4 (2–10) | 7 (3–14.5) | 0.04 |
| Infections | 5 (2–10) | 7 (3–15) | 0.04 |
| Contact material | 7 (3–10) | 6 (3–14) | 0.65 |
| Stress | 5 (3–10) | 7 (3–15) | 0.22 |
| Generalised urticaria | 7 (3–14) | 7 (3–12) | 0.95 |
| Angio-oedema | 7 (3–15) | 7 (2–12) | 0.37 |
| SPT positivity | 3 (1.5–7.5) | 5 (3–10) | 0.47 |
| Mite | 2 (1–7) | 5 (3–10) | 0.34 |
| Pollen | 1.5 (1–5) | 5 (3–12) | 0.02 |
| Cat | 5 (3–7) | 5 (2–10) | 0.97 |
| Dog | 1 (1–2) | 5 (3–10) | 0.02 |
| Food | 5 (2–8) | 4 (2–10) | 0.99 |
| Steroid treatment | 6 (3–10) | 7 (2–15) | 0.52 |
ACE: angiotensin converting enzyme, SPT: skin prick test.
The AU patients in the retrospective data subgroup were compared to those in the prospective data subgroup and there were no significant differences in age, gender, the number of previous attacks of AU, chronic disease and drug use, potential triggers of attacks of AU, asthma, drug allergy, metal allergy, bee venom allergy, the presence of generalised urticaria and angio-oedema, or SPT positivity. More patients in the prospective data subgroup had a history or rhinitis and food allergy than in the retrospective data (25.7% vs. 10.6% (p=0.003) and 8.1% vs. 2.4% (p=0.04), respectively). More patients in the retrospective data subgroup were treated with systemic steroids and mean attack duration was longer in the retrospective data subgroup than in the prospective data subgroup (60.9% vs. 45.9% (p=0.03) and 11.2d vs. 5.1d (p=0.002), respectively).
The CU group had a significantly higher prevalence of non-steroidal anti-inflammatory drug hypersensitivity (NSAIDH) and antibiotic hypersensitivity (24.7% vs. 4.3% (p<0.01) and 10.6% vs. 4.6% (p<0.01), respectively) based on history and/or provocation test results, and a history of allergic reactions to foods (18.3% vs. 3.9% (p<0.01)) than the AU group (Table 5). Multiple linear regression analysis that included all these significant factors showed that NSAIDH (OR: 7.97; 95%CI: 4.33–14.66; p<0.01) and food allergy (OR: 5.17; 95%CI: 2.71–9.85; p<0.01) were associated with CU.
Univariant analysis of differences between chronic and acute urticaria patients.
| Acute urticaria | Chronic urticaria | p | |
| (n=281) | (n=953) | ||
| Age, mean (SD) | 36.66 (14.26) | 37.28 (12.24) | 0.48 |
| Acute urticaria | Chronic urticaria | p | ORc (95%CI) | |
| n (%) | n (%) | |||
| Male gender | 93 (33.1) | 288 (30.2) | 0.35 | 0.88 (0.68–1.16) |
| Chronic disease | 79 (28.1) | 256 (26.8) | 0.66 | 0.93 (0.69–1.26) |
| Asthma | 23 (8.2) | 98 (10.4) | 0.28 | 1.29 (0.81–2.08) |
| Rhinitis | 41 (14.6) | 185 (19.4) | 0.07 | 1.41 (0.97–2.04) |
| NSAID hypersensitivitya | 12 (4.3) | 236 (24.7) | <0.01 | 7.39 (4.17–14.28) |
| Antibiotic hypersensitivitya | 13 (4.6) | 101 (10.6) | <0.01 | 2.44 (1.35–4.35) |
| Food allergy | 11 (3.9) | 174 (18.3) | <0.01 | 5.49 (2.94–10.00) |
| Metal allergy | 42 (14.9) | 139 (14.6) | 0.85 | 0.97 (0.67–1.41) |
| Venom allergy | 5 (1.8) | 8 (0.8) | 0.17 | 0.47 (0.15–1.45) |
| Familial atopy | 109 (38.8) | 389 (40.8) | 0.55 | 1.08 (0.82–1.43) |
| Positive SPTb | 18 (26.5) | 114 (33.2) | 0.28 | 1.38 (0.77–2.50) |
AU occurs more frequently in females and at young to middle ages,5,9 as in the present study. We identified the general characteristics of AU patients and the potential triggers of attacks of AU, and observed that the history-based frequency of possible drug-related aetiology (38.1%) was higher than previously reported (6.3–9.2%).4,5 The most commonly reported drugs used were NSAIDs and antibiotics; among the antibiotics used, beta-lactams were the most common, which is in agreement with the literature.10 Oral provocation tests were performed in 21.5% of the AU patients with a suspicious drug history; however, none of the tests performed to confirm drug hypersensitivity were positive. It was reported that only 21.1% of patients with a clinical history suggestive of beta-lactam allergy actually had an allergy confirmed by skin tests or drug provocation tests.11 NSAIDs are another group of drugs that are widely known to cause urticaria and/or angio-oedema, either via an IgE-mediated reaction or inhibition of the enzyme cyclooxygenase 1.12 In the present study infections accompanied drug use in 63.5% of the AU patients with a possible drug-related aetiology. It is likely that infections – not the drugs used for treatment – were the triggering factor for attacks of AU in these patients. The actual rate of drug-related AU might have been much lower than the observed rate had all the patients with a suspicious drug intake history undergone oral provocation tests.
Infections were the second most common potential trigger of attacks of AU in the present study, occurring in 35.2% of the patients; upper respiratory tract infections were the most common. The observed frequency rate is similar to that reported in other studies.4,5 Furthermore, infections might have been the most common aetiology in the present study had all the patients with a suspicious drug intake history undergone oral provocation tests. The duration of attacks of AU was significantly shorter in the patients in which infection was a triggering factor. Kulthanan et al.5 reported that most patients with infection-related AU had complete remission within three weeks.
Suspicious food intake was reported by 17.8% of the present study's AU patients. The most common suspected foods were fruits, vegetables, and fresh fruit juices. Foods were reported to be the possible cause of attacks of AU in 0.9% and 1.3%, respectively, of patients in two previous studies4,5; however, Juhlin reported that foods and drinks were associated with exacerbation of wheals in 30% and 18%, respectively, of patients with recurrent attacks of urticaria.13 Self-reported allergy to vegetables and fruits is common, ranging from 0.01% to 13.7% and 0.02% to 8.5%, respectively, but following food challenge these prevalences decrease significantly to 0.1–0.3% for vegetables and 0.1–4.3% for fruits.14 In the present study SPTs for foods were performed in 16 of the 50 AU patients with a history of allergic reactions to foods, but none had a positive result; however, food challenges were not performed, which is a limitation of the study. The actual rate of food-related AU might have been much lower than the self-reported rate had food challenges been performed. In the present study attacks of AU in the patients with suspicious food intake were of shorter duration than in those without suspicious food intake.
Stress was reported as a triggering factor for attacks of AU by 24.7% of the present study's patients. The role of stress in AU has not been previously studied, but some studies reported that there is a relationship between emotional stress and the onset or exacerbation of CU.15,16 Psychological stress was evaluated by asking the patients in the present study if they had experienced a major life event in the recent past. The questions related to stress used in the present study were not standardised or structured, which could be considered another limitation. We think that the role of stress as a triggering factor in attacks of AU should be evaluated in greater detail using better quantification methods, such as standardised questionnaires.
In addition to infections and foods, in the present study patients with a history of rhinitis and food allergy, and pollen and dog sensitivity based on SPTs had attacks of shorter duration than the patients without these characteristics. One explanation for this finding might be that the occurrence of IgE-mediated urticarial reactions was more common in these patients. In contrast, patients with a history of asthma had attacks of longer duration than those without a history of asthma. Lin et al. studied 1075 children with AU and reported that patients with a history of allergic diseases had attacks of longer duration than those without a history of allergic diseases.17
The relationship between atopic diseases and urticaria was investigated in many studies. Juhlin13 reported that a history of rhinitis, asthma, or atopic dermatitis was observed in more than one-third of the 330 patients with recurrent urticaria. Asthma was noted in 11% and rhinitis in 20% of the patients in that study. In another study that included 390 urticaria patients, 26% had hay fever, asthma, or atopic eczema, or >1 of these conditions.18 Asero and Madonini19 reported that among 26 CU patients, 8% had asthma and 77% had bronchial hyperresponsiveness. A study that included 562 patients with episodic urticaria, CU, and AU reported that the prevalence of rhinitis and asthma was 14.5% and 2.8%, respectively.20 An earlier study conducted in Turkey reported that the prevalence of asthma and rhinitis was 6.2–11.2% and 11.7–21.2%, respectively21; the prevalence of asthma and rhinitis in the AU and CU patients in the present study was similar to that in the general population. Comparison of the AU and CU groups showed that the prevalence of asthma and rhinitis was higher in the CU group, but not significantly.
By comparing AU and CU patients we aimed to determine if there were differences in their demographic and clinical characteristics, and if there are some factors that could be used to predict if patients with AU will progress to CU. We observed that the prevalence of NSAIDH based on history and/or provocation test results was significantly higher in the CU group than in the AU group (24.7% vs. 4.3%), which was also the factor most strongly associated with CU. In previous studies 22–26% of patients with chronic idiopathic urticaria experienced exacerbation following aspirin challenge.22,23 A history of food allergy in the present study's CU group was also significantly more common than in the AU group, which might have been due to the ingestion of foods and food additives containing natural salicylates in the patients with NSAIDH, but logistic regression analysis showed that food allergy history was an independent risk factor for CU. The prevalence of antibiotic hypersensitivity based on history and/or provocation test results was also significantly higher in the present study's CU group, but logistic regression analysis showed that antibiotic hypersensitivity was not an independent risk factor for CU, which might have been due to the higher prevalence of antibiotic hypersensitivity in the presence of NSAIDH, as previously reported.24,25
The present study has some limitations. Ideally, the possible triggers of attacks of AU, especially drugs, foods, and stress, should have been investigated prospectively using more objective methods; but according to current guidelines1 routine diagnostic tests or an extended diagnostic programme are not recommended in acute spontaneous urticaria unless strongly suggested by patient history such as allergy. Data about the epidemiology of AU are lacking in the literature and the present study does have some novel aspects in this respect. We investigated the factors that affected the duration of attacks of AU in an adult population. Additionally, to the best of our knowledge the present study is the first to identify some predictive factors for progression from AU to CU. We observed that there may be some patient-related factors associated with CU such as NSAIDH or food allergy. We believe that identification of AU patients who have a high risk of progression to CU is important since these patients need a better follow-up.
The present study, which is primarily a history-based epidemiological survey, should be considered a preliminary study of the natural course of attacks of AU. Additional research based on more objective methods is necessary to more clearly determine the aetiology of AU, and the factors that affect the duration of attacks of AU and their mechanisms of action. We think that a good quality standardised questionnaire, together with physical examination and appropriate laboratory tests, can provide important clues related to the progression from AU to CU and guide the follow-up of AU patients.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received
Right to privacy and informed consentRight to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestAll authors declare that there is no conflict of interest and no funding.
We would like to thank Ahmet U. Demir, Associate Professor, Hacettepe University Medical Faculty, Department of Chest Disease, for statistical analysis of the data and Bulent Erbil, MD, Hacettepe University Medical Faculty, Adult Emergency Department, for data collection and patient referral.