Emerging evidence suggests that sex steroid hormones may influence respiratory symptoms. The existing literature about the role of oral contraceptive pill (OCP) on respiratory disease is scarce and conflicting especially during the adolescent period. In this study, we aimed to investigate the effect of OCPs on current wheezing among adolescents and young adults.
MethodsA questionnaire was administered face-to-face to adolescents and young women by a physician. The questionnaire included ISAAC survey-comprised questions on ever wheezing, current wheezing, allergic diseases, smoking history (active or passive), and family history of allergic diseases and questions on OCP usage status. The effect of OCPs on wheezing was evaluated by logistic regression analysis.
ResultsA total of 487 subjects aged between 11.3 and 25.6years participated in the study and 196 (40.2%) reported that they had used OCPs. 7.4% of the participants had physician-diagnosed asthma and 10.3% of them were active smokers. It was detected that OCPs were associated with increased risk for current wheezing (odds ratio, 2.36; 95% CI, 1.25–4.47 adjusted for asthma and current smoker) and this risk was related with the usage during the past year.
ConclusionYoung women taking oral contraceptives had a higher rate of current wheezing, suggesting that sex steroids may be of importance for respiratory health.
The prevalence of asthma is higher in adolescent girls and adult women, although it is higher in males during the first decade of life.1–3 Severe asthma is more prevalent in adult women; asthma attacks may be triggered by menarche, and the prevalence and severity of asthma decrease after menopause.4–6 These findings suggest that oestrogens and progestins could play a role in asthma pathogenesis. Oral contraceptive pills (OCPs) contain oestrogens and progestins and are widely used in contraception and other medical indications all over the world.
The existing literature about the role of OCPs on respiratory disease is scarce and conflicting and these studies are mainly carried out in adults. It has been reported that OCPs were associated with increased risk for asthma and wheezing but there was not any significant increase in asthma risk among women using OCPs in the Copenhagen City Heart Study.3,7,8 Forbes et al. did not observe an increase in asthma severity in OCPs users.9 Salam et al. showed that OCP was associated with more wheeze among subjects without asthma.10
OCPs are also widely used during the adolescent period for indications such as irregular menstruation, hirsutism, polycystic ovarian disease, dysmenorrhoea and contraception; however, there are very few studies for this age group. In this study, we aimed to define the effect of OCPs on current wheezing among adolescents and young adults.
MethodsStudy population and study designThis cross-sectional study was performed consecutively on female patients admitted to Youth Center of Ankara Zekai Tahir Burak Women's Health, Education and Research Hospital which serves patients aged from menarche to 25years between May and September 2010. Exclusion criteria were: (1) being pregnant at that time, (2) not having menstruations yet, and (3) refusing to enter the study. The study was approved by the institutional review board of Ankara Children's Hematology Oncology Education and Research Hospital, and written informed consent was obtained from the participants.
Study designA questionnaire was administered face-to-face to each participant selected consecutively by the same fellow of the paediatric allergy and immunology fellowship just before the gynaecology visit. The study was presented as a survey about allergic diseases, and asthma and allergic rhinitis were defined on self-report of physician diagnosis during the subject's lifetime.
QuestionnaireThe questionnaire consisted of two major parts. The first part was developed mainly based on ISAAC survey-comprised questions on ever wheezing, current wheezing, allergic diseases, smoking history (active or passive), and family history of allergic diseases.11 Answers given as “yes” to the following questions: “Have you ever had wheezing or whistling in the chest any time in the past?” and “Have you had wheezing or whistling in the chest in the past 12 months?” were accepted as having ever wheezing and current wheezing, respectively. All participants were asked about the prevalence of physician-diagnosed asthma and allergic rhinitis. Smoking history was assessed through questioning on active smoking status, second-hand smoking and exposure to smoke in the first year of life. We also assessed the socioeconomic status (parental education, annual family income, etc.) and prevalence of allergic diseases in family members. The presence of allergic diseases was based on self-report of physician diagnosis of asthma and allergic rhinitis.
The second part of the questionnaire covered the age at menarche, history of OCP use, including duration and time period. Oral contraception was defined with the answer “yes” to the question, “Have you ever used OCP up to now?” Subjects were categorised into two main groups: non-users and users. Ever users were also divided into two subgroups; those who used in the past year and those who used longer than a year ago. All users were asked for the indications of OCPs and non-users were also asked about their diagnosis made by gynaecologists at the current visit.
All the participant's weight and height were measured, and body mass index (BMI) was calculated as weight in kilos divided by per square of height in metres.
Statistical analysisStatistical analyses included frequency and percent distributions, calculation of prevalence rates for CW and potential risk factors, and group comparisons using chi-squared, Student's t-tests and Mann–Whitney U test as appropriate.
To examine the association between OCP use and current wheezing, initially, the crude association between risk factors and CW was analysed. Variables that were associated with the outcomes in the univariate analysis at a p-value of <0.25 were examined in the multivariate logistic regression models. Final models were achieved by Backward LR elimination method to identify the most parsimonious, yet statistically significant; adjusted odds ratios (ORs) and relevant 95% confidence intervals (CIs) are presented in tables. The analysis was conducted using the statistical software program the Statistical Package for the Social Sciences, version 15.0 (SPSS, Inc., Chicago, IL, USA).
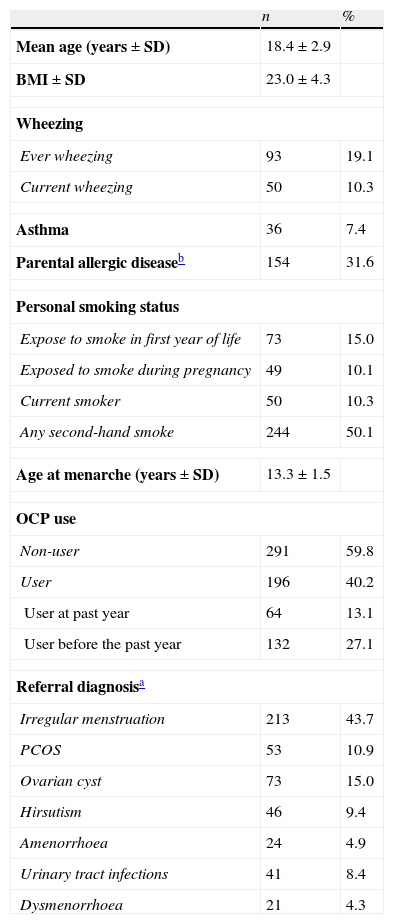
ResultsA total of 487 females between 11.3 and 25.6years of age accepted to participate in the study (18 patients refused to enter the study). Their mean age was 18.4±0.1years and all the participants were unmarried and non-pregnant. Of 487 young women, 196 (40.2%) reported that they had taken OCPs. Prevalence of ever wheezing and current wheezing in all participants was 19.1% and 10.3%, respectively. General characteristics of the participants are given in Table 1.
Characteristics of participants (N=487).
| n | % | |
| Mean age (years±SD) | 18.4±2.9 | |
| BMI±SD | 23.0±4.3 | |
| Wheezing | ||
| Ever wheezing | 93 | 19.1 |
| Current wheezing | 50 | 10.3 |
| Asthma | 36 | 7.4 |
| Parental allergic diseaseb | 154 | 31.6 |
| Personal smoking status | ||
| Expose to smoke in first year of life | 73 | 15.0 |
| Exposed to smoke during pregnancy | 49 | 10.1 |
| Current smoker | 50 | 10.3 |
| Any second-hand smoke | 244 | 50.1 |
| Age at menarche (years±SD) | 13.3±1.5 | |
| OCP use | ||
| Non-user | 291 | 59.8 |
| User | 196 | 40.2 |
| User at past year | 64 | 13.1 |
| User before the past year | 132 | 27.1 |
| Referral diagnosisa | ||
| Irregular menstruation | 213 | 43.7 |
| PCOS | 53 | 10.9 |
| Ovarian cyst | 73 | 15.0 |
| Hirsutism | 46 | 9.4 |
| Amenorrhoea | 24 | 4.9 |
| Urinary tract infections | 41 | 8.4 |
| Dysmenorrhoea | 21 | 4.3 |
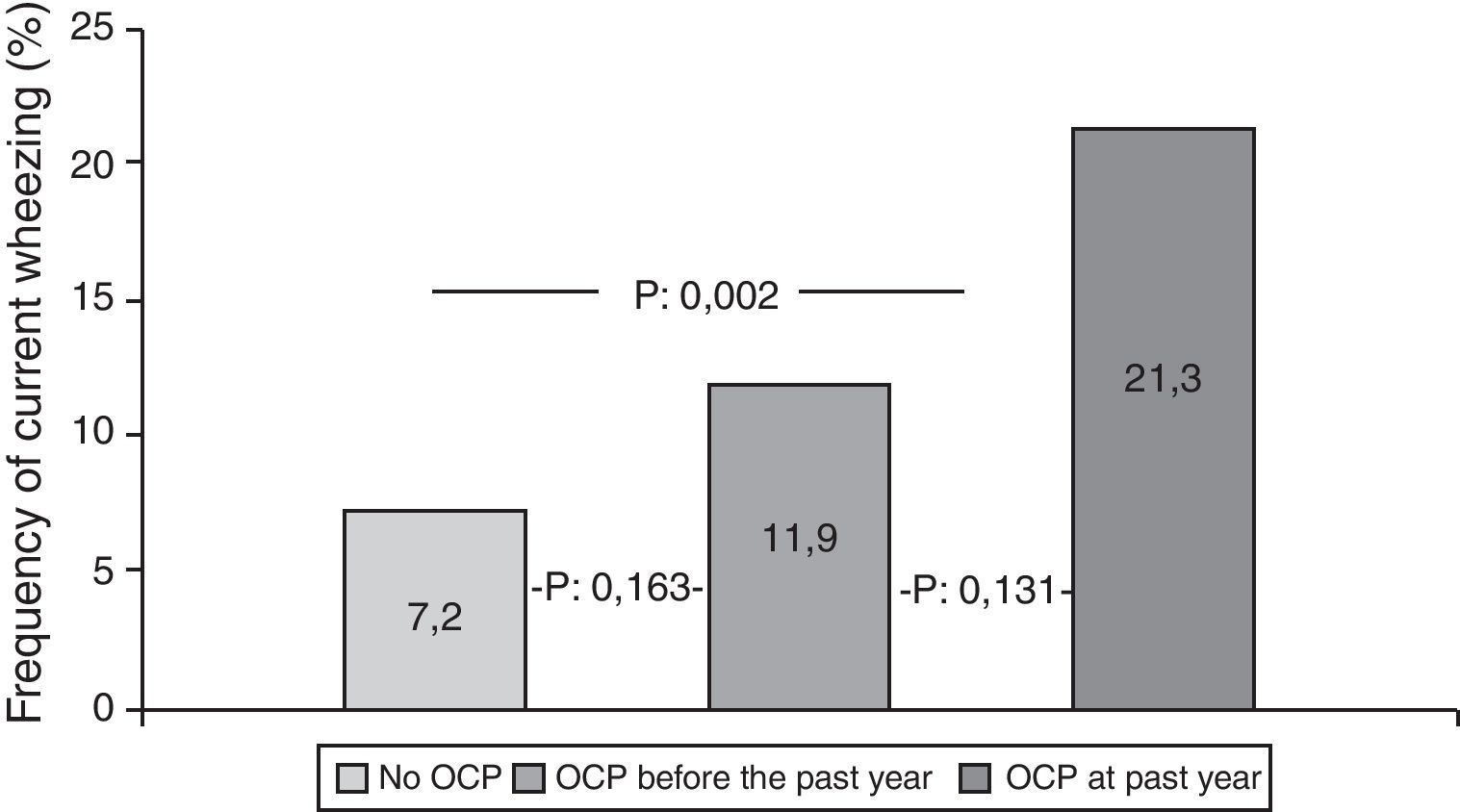
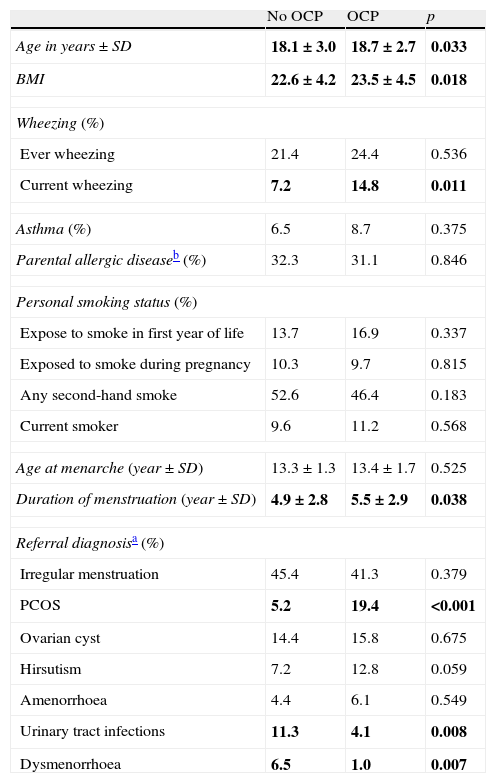
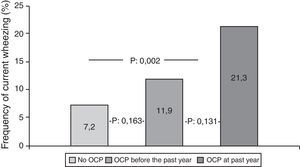
Participants who used OCPs are slightly older and have higher BMI than non-users. Although the most common diagnosis was irregular menstruation in both groups (41.3% and 45.4%, respectively), PCOS prevalence was significantly higher in OCP users. There were no significant differences in the prevalence of asthma, ever wheezing, parental allergic disease, or smoking habits between the two groups. There was no relation between ever wheezing and OCPs, whereas the prevalence of current wheezing was significantly higher in the OCP user group compared to non-users (14.8% and 7.2%, p:0.011). Characteristics of groups are shown in Table 2. Further analyses showed that the increased risk for current wheezing was related to the OCP usage in the last year rather than usage before this period (Fig. 1).
Comparison of age, asthma, parental allergic disease, smoking status, age at menarche and referral diagnosis according OCP use.
| No OCP | OCP | p | |
| Age in years±SD | 18.1±3.0 | 18.7±2.7 | 0.033 |
| BMI | 22.6±4.2 | 23.5±4.5 | 0.018 |
| Wheezing (%) | |||
| Ever wheezing | 21.4 | 24.4 | 0.536 |
| Current wheezing | 7.2 | 14.8 | 0.011 |
| Asthma (%) | 6.5 | 8.7 | 0.375 |
| Parental allergic diseaseb (%) | 32.3 | 31.1 | 0.846 |
| Personal smoking status (%) | |||
| Expose to smoke in first year of life | 13.7 | 16.9 | 0.337 |
| Exposed to smoke during pregnancy | 10.3 | 9.7 | 0.815 |
| Any second-hand smoke | 52.6 | 46.4 | 0.183 |
| Current smoker | 9.6 | 11.2 | 0.568 |
| Age at menarche (year±SD) | 13.3±1.3 | 13.4±1.7 | 0.525 |
| Duration of menstruation (year±SD) | 4.9±2.8 | 5.5±2.9 | 0.038 |
| Referral diagnosisa (%) | |||
| Irregular menstruation | 45.4 | 41.3 | 0.379 |
| PCOS | 5.2 | 19.4 | <0.001 |
| Ovarian cyst | 14.4 | 15.8 | 0.675 |
| Hirsutism | 7.2 | 12.8 | 0.059 |
| Amenorrhoea | 4.4 | 6.1 | 0.549 |
| Urinary tract infections | 11.3 | 4.1 | 0.008 |
| Dysmenorrhoea | 6.5 | 1.0 | 0.007 |
P values<0.05 were written in bold.
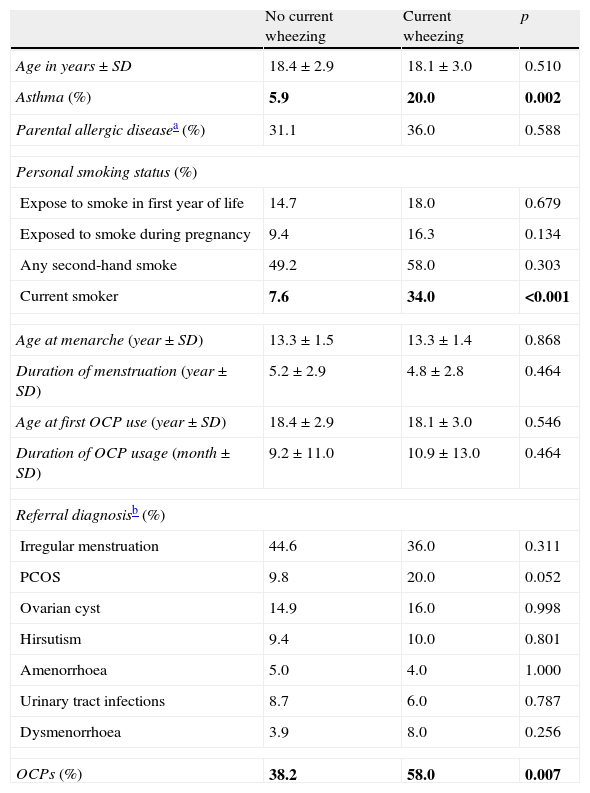
We also compared the groups with and without current wheezing. Subjects with current wheezing were more often active smokers and used OCPs than subjects without current wheezing. There was no difference regarding referral diagnosis between these groups (Table 3).
Comparison of age, asthma, parental allergic disease, smoking status, age at menarche, OCP and referral diagnosis according presence of current wheezing.
| No current wheezing | Current wheezing | p | |
| Age in years±SD | 18.4±2.9 | 18.1±3.0 | 0.510 |
| Asthma (%) | 5.9 | 20.0 | 0.002 |
| Parental allergic diseasea (%) | 31.1 | 36.0 | 0.588 |
| Personal smoking status (%) | |||
| Expose to smoke in first year of life | 14.7 | 18.0 | 0.679 |
| Exposed to smoke during pregnancy | 9.4 | 16.3 | 0.134 |
| Any second-hand smoke | 49.2 | 58.0 | 0.303 |
| Current smoker | 7.6 | 34.0 | <0.001 |
| Age at menarche (year±SD) | 13.3±1.5 | 13.3±1.4 | 0.868 |
| Duration of menstruation (year±SD) | 5.2±2.9 | 4.8±2.8 | 0.464 |
| Age at first OCP use (year±SD) | 18.4±2.9 | 18.1±3.0 | 0.546 |
| Duration of OCP usage (month±SD) | 9.2±11.0 | 10.9±13.0 | 0.464 |
| Referral diagnosisb (%) | |||
| Irregular menstruation | 44.6 | 36.0 | 0.311 |
| PCOS | 9.8 | 20.0 | 0.052 |
| Ovarian cyst | 14.9 | 16.0 | 0.998 |
| Hirsutism | 9.4 | 10.0 | 0.801 |
| Amenorrhoea | 5.0 | 4.0 | 1.000 |
| Urinary tract infections | 8.7 | 6.0 | 0.787 |
| Dysmenorrhoea | 3.9 | 8.0 | 0.256 |
| OCPs (%) | 38.2 | 58.0 | 0.007 |
P values<0.05 were written in bold.
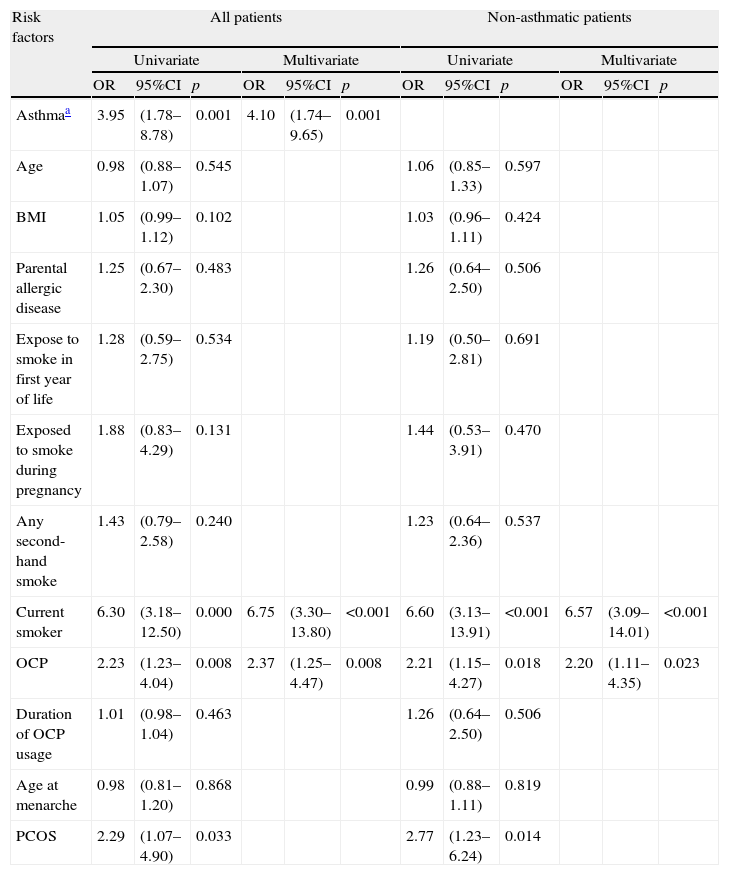
We observed a positive association between OCP usage and current wheezing with asthma diagnosis and active smoking after adjusting for asthma, age, smoking habits (active, second-hand smoking and exposure to smoke during first year of life), BMI, parental allergic disease, age at menarche, duration of OCP usage and referral diagnosis; 2.36 (1.25–4.47, p:0.008) [OR(%95 CI)] (Table 4). We also made multiple regression analyses after excluding the asthmatic patients in order to rule out the mixing effect of asthma. We found that the positive correlation between OCP and current wheezing still persisted after the adjustments for asthma, age, smoking habits, BMI, parental allergic disease, age at menarche, duration of OCP usage and referral diagnosis 2.19 (1.11–4.34, p:0.023) [OR(95% CI)] (Table 4). As PCOS is a strong indication of OCP and there was a high strength of association of wheezing with smoking, we also made analysis in those without PCOS and the non-smoking group separately. We also found that both in the non-smoking group and in those without PCOS the positive correlation between OCP and current wheezing still persisted [3.04 (1.41–6.53, p:0.004); 2.02 (1.01–4.05, p:0.048), respectively]. Furthermore, we did not find a relation between the OCP and current smoking (p:0.568).
The associations between risk factors and current wheezing.
| Risk factors | All patients | Non-asthmatic patients | ||||||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| Asthmaa | 3.95 | (1.78–8.78) | 0.001 | 4.10 | (1.74–9.65) | 0.001 | ||||||
| Age | 0.98 | (0.88–1.07) | 0.545 | 1.06 | (0.85–1.33) | 0.597 | ||||||
| BMI | 1.05 | (0.99–1.12) | 0.102 | 1.03 | (0.96–1.11) | 0.424 | ||||||
| Parental allergic disease | 1.25 | (0.67–2.30) | 0.483 | 1.26 | (0.64–2.50) | 0.506 | ||||||
| Expose to smoke in first year of life | 1.28 | (0.59–2.75) | 0.534 | 1.19 | (0.50–2.81) | 0.691 | ||||||
| Exposed to smoke during pregnancy | 1.88 | (0.83–4.29) | 0.131 | 1.44 | (0.53–3.91) | 0.470 | ||||||
| Any second-hand smoke | 1.43 | (0.79–2.58) | 0.240 | 1.23 | (0.64–2.36) | 0.537 | ||||||
| Current smoker | 6.30 | (3.18–12.50) | 0.000 | 6.75 | (3.30–13.80) | <0.001 | 6.60 | (3.13–13.91) | <0.001 | 6.57 | (3.09–14.01) | <0.001 |
| OCP | 2.23 | (1.23–4.04) | 0.008 | 2.37 | (1.25–4.47) | 0.008 | 2.21 | (1.15–4.27) | 0.018 | 2.20 | (1.11–4.35) | 0.023 |
| Duration of OCP usage | 1.01 | (0.98–1.04) | 0.463 | 1.26 | (0.64–2.50) | 0.506 | ||||||
| Age at menarche | 0.98 | (0.81–1.20) | 0.868 | 0.99 | (0.88–1.11) | 0.819 | ||||||
| PCOS | 2.29 | (1.07–4.90) | 0.033 | 2.77 | (1.23–6.24) | 0.014 | ||||||
BMI: body mass index; OR: odds ratio; PCOS: polycystic ovarian syndrome.
We observed that OCPs were associated with increased occurrence of current wheezing among adolescents and young females. The relation between OCPs and current wheezing was only observed in subjects who had taken the OCPs in the previous year, whereas it was not observed in subjects who had taken them more than a year ago. We also showed that the effect of OCPs on current wheezing persists in non-asthmatic and non-smoking patients separately.
It has been seen that there is a similar increase in the prevalence of asthma and frequency of women using OCPs in the last 2–3 decades. It has been reported that maternal previous use of OCPs before pregnancy might increase the risk of allergic airway disease among offspring compared with those children whose mothers had not used OCPs.12,13 These suggestions give rise to the thought that women using these pills may be at the same risk for airway diseases. While Macsali et al. reported that OCPs were associated with increased risk for asthma and asthma symptoms, Salam et al. showed that OCP use was associated with a decrease in current wheeze in woman with a history of asthma, whereas women without asthma had more wheeze when using OCP.8,10 However, Copenhagen City Heart Study showed that OCPs have no effect on the prevalence of asthma, which shows us that the relationship between OCPs and asthma is conflicting.7 Additionally, our results are conflicting with an apparent protective role of OCP in asthma, suggested by the Tasmanian Asthma Survey.14 The underlying mechanism is probably mediated by the increased levels of oestrogen and progesterone. However, OCPs act by decreasing the levels of these hormones by blocking ovulation. The reason why the risk of current wheezing is high in non-asthmatics after OCP usage may be related to the decreased relaxing effects of both progesterone and oestrogen on bronchial smooth muscle, because the levels of these sex steroids will be lower after contraception.
Animal models suggest that both progesterone and oestrogen may directly affect the lungs by reducing contractility and increasing relaxation of bronchial smooth muscle.15,16 It has also been shown that female sex hormones have a dual effect on allergic lung inflammation, which are being inflammatory and causing eosinophil recruitment to the lungs and being anti-inflammatory and reducing cell migration.17 Kirsch et al. also showed that oestrogens stimulate endothelial nitric oxide synthase which is an important mediator of physiologic process in the airways.18 Ethinylestradiol which is the most common oestrogen in OCPs is a very potent oestrogen and might have direct inflammatory effects on airways. Hormonal fluctuations during the menstrual cycle are associated with worsening of asthma symptoms and the predominance of TH2-mediated immunity during the perimenstrual period when the levels of sex steroids are low.19,20 Numerous case studies suggest that premenstrual declines in hormonal levels worsen asthma symptoms, and that exogenous hormone therapy, in reducing hormonal fluctuation, eliminates premenstrual asthma exacerbations and potentially reduces or eliminates corticosteroid dependence.21,22 Beneficial effects of treatment with larger doses of progesterone were seen in women with severe premenstrual asthma.23 Furthermore, it has been shown that asthmatic women had a longer period of low progesterone levels than women without asthma.24 OCPs inhibit the natural surge in progesterone in the lutheal phase by the 20-fold lower daily amounts of progesterone. Therefore, it may be speculated that one of the factors that may influence current wheezing is the low level of progesterone during OCP treatment.
In our study, active smoking was found to be the strongest risk factor for current wheezing. Recent studies conducted in adolescents also reported that active smoking increases the risk of current wheezing and asthma symptoms.25,26 Because of the strong relation between active smoking and current wheezing, we analysed the relation between OCPs and current wheezing in non-smokers separately. We found that the increasing effect of OCPs on current wheezing also persisted in this group.
The majority of the participants had menstrual disorders, leading us to consider hormonal imbalance, and PCOS diagnosis was frequent with respect to the OCP user group. As PCOS is a strong indication of OCP we also made an analysis in those without PCOS and the positive correlation between OCP and current wheezing still persisted. Although neither the available literature nor our study puts forward any relation between PCOS and current wheezing, it has been known that testosterone, which is highly increased in patients with PCOS, has also been associated with relaxation of bronchial tissue and depressed response to histamine.16 In accordance with this information, OCPs might increase the prevalence of current wheezing via their depressing effects on androgen levels in patients with PCOS.
Obesity is another important factor that is related to current wheezing. Tucson Children's Respiratory Study showed a statistically significant positive association between obesity and wheezing in women who reached puberty under 11years whereas obesity was not associated with wheezing when puberty occurs after 11years of age.27 In our study the mean age of subjects at menarche was 13.2years and we detected that there was no relation between the BMI and current wheezing in logistic regression analysis.
The major limitations of this study are the use of a cross-sectional survey with self-reported asthma and OCP. There might be information bias especially in under reporting of oral contraceptive use. In order to decrease that effect we presented the study as a study about allergic diseases in general. This might also cause a selection bias towards those having allergic disease. However the prevalence of asthma within our participants was similar to that of the general population (7.4%). Moreover, analysis was carried out in the non-asthmatic group separately in order to decrease the effect of asthma and only 18 patients refused to enter the study. It is also difficult to interpret the time sequence of exposure and outcome in cross-sectional studies. Although prospective studies are the best way to determine this relationship, due to the persisted effect of OCPs on current wheezing after adjustment for risk factors, OCP influencing the airways seems to be the most likely explanation of wheezing and OCP in our study. In this cross-sectional study we accepted subjects with asthma on the basis of self-reported physician-diagnosed asthma. However, self-reported asthma has been found to reflect physician diagnosis accurately and it has been widely used in epidemiological studies.28 But interviews with participants were conducted face-to-face by a physician, which makes our finding stronger and more reliable. Because of the low numbers of asthmatic patients in our study group, we could not make risk analysis on the asthmatic group separately. This prevented us from comparing the effect of OCPs on asthmatic and non-asthmatic patients.
ConclusionIn conclusion, young women taking oral contraceptives had a higher rate of current wheeze, suggesting that sex steroids may of importance for respiratory health. The findings originate from a cross-sectional survey and should be interpreted with caution and there is a requirement to conduct wider scope prospective studies aimed at demonstrating the relation between OCP and asthma.
Ethical disclosuresPatients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest to declare.