To investigate neonatal maternal separation (NMS) effects on airway inflammation of asthma and potential mechanism using a mouse model.
Methods80 Balb/c neonatal male mice were randomly assigned to NMS and non-NMS groups. Feces were collected on PND21, 28, 35 and 42 to analyze microbiota and short-chain fatty acids (SCFAs). Non-NMS group were then divided into control (group A) and asthma groups (group B), while NMS group was assigned to NMS+asthma (group C) and NMS+SCFAs+asthma groups (group D). Inflammatory cells and eosinophils (EOS) in bronchoalveolar lavage fluid (BALF) were assessed. Pathological changes and cytokines in lung tissue were observed. Protein expression of Occludin and E-cadherin in airway epithelial was examined.
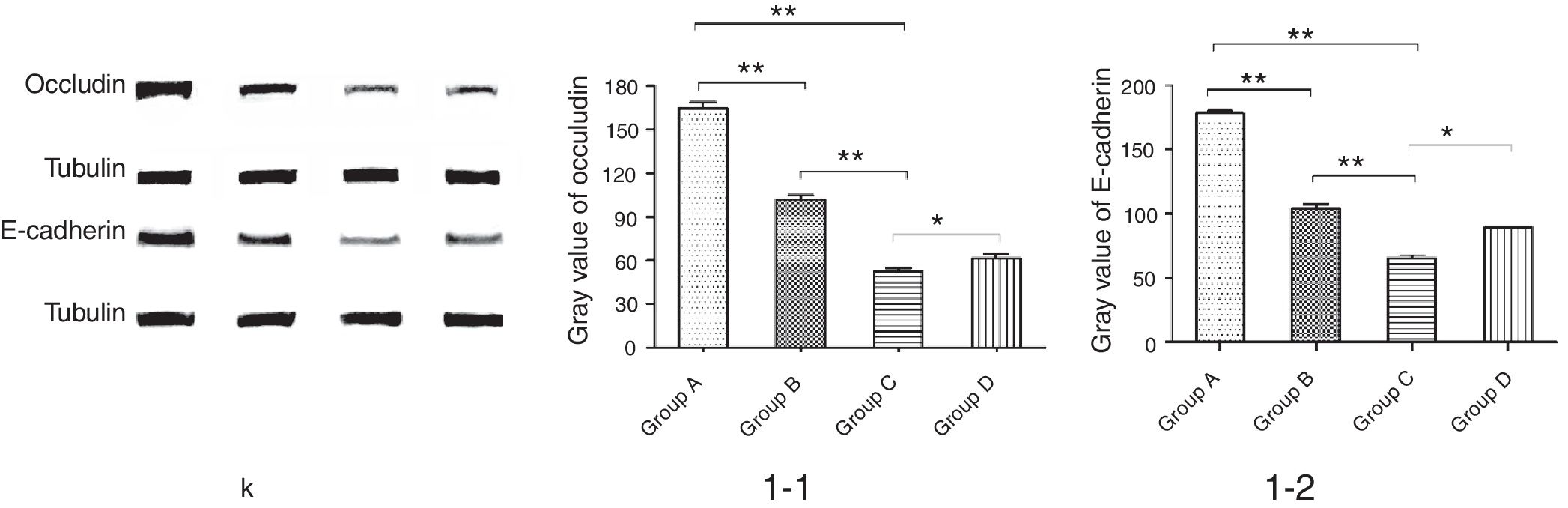
ResultsThe number of S′, diversity index H′ and dominance index D′, as well as content butyric acid in NMS group C were significantly lower than non-NMS group B (p<0.05). Mice in group C had a higher level of inflammatory cells and EOS compared with group A, B and D. EOS moderate infiltration was found in mice of group B, C and D. Mice in group C had significantly higher levels of cytokines and showed slightly increased bronchial epithelium goblet cells and a small amount of visceral secretions. Occludin and E-cadherin expression in lung in B, C and D groups was depressed, and protein level in group C was significantly lower than group B and D.
ConclusionsNMS is associated with exacerbated inflammation of adult asthma by changing intestinal microflora resulting in butanoic acid decline and airway epithelial barrier damage.
Early life stresses refer to the various negative life events encountered before adulthood,1 and these are risk factors for a variety of diseases which often affect the growth and development of children. Studies have shown that babies in early life are sensitive to these stresses such as using antibiotics at late pregnancy, sound stimulation, etc.), which can influence brain development, lung structure and function, gastrointestinal and immune function. These changes may continue to adult,2–4 and not only lead to autism, depression, irritable bowel syndrome and other diseases,5–7 but also increase asthma severity.
Neonatal maternal separation (NMS) is a common early life stress event.5 At present, it has been proved that NMS can cause the offspring anxiety and depression, and also induce irritable bowel syndrome.7 But there is limited study showing the relationship between NMS and asthma in adults.
In this study, we referred NMS as the early life stress event and observed the change of the intestinal flora, the content of short-chain fatty acids (SCFAs) and the severity of asthma in adult male mice.
Materials and methodsAnimalsSixteen female and eight male Balb/c mice (20–23g) were obtained from Nanjing Qinglong Laboratory Animal, China. Each male mouse was housed with two female mice for mating before the experiment. Pregnant mice were housed one per cage in a quiet environment, maintained at 20±1°C with a 12-h light/12-h dark cycle, and received SPF food and water ad libitum. The environmental temperature was kept constant, and relative humidity was 50–60%. All experiments were approved by the Animal Care Committee of Southeast University and conducted in accordance with the guidelines of the Care and Use of Laboratory Animals.
Neonatal maternal separationAfter delivery, the maternal mice were housed with their 8–12 babies. All of them were maintained in 22cm×9cm×15cm plastic cages in the environment as mentioned above. In consideration of the variable and uncontrollable factors of female mice, such as the hormone cycles, male mice were chosen to perform this research. NMS mouse model was established as described by O’Mahony et al.7 Eighty litters were randomly assigned into NMS group (n=40) and non-NMS group (unhandled controls, n=40). Mice in the NMS group were removed carefully from their cages to other prepared cages in another room with the same condition for 3h daily (from 8:00AM to 11:00AM) during the same time each day on postnatal day (PND, data of birth as PND0) 2–14. Non-NMS mice remained undisturbed with their mothers in the cages. On PND21, mice in the non-NMS group were divided into two groups randomly: the normal control group (group A) and asthma group (group B); while mice in NMS group were randomly assigned into NMS+asthma group (group C) and NMS+SCFAs+asthma group (group D). Mice in group D were feed with SCFAs (200mmol/l of butyric acid) from PND21 to PND42.
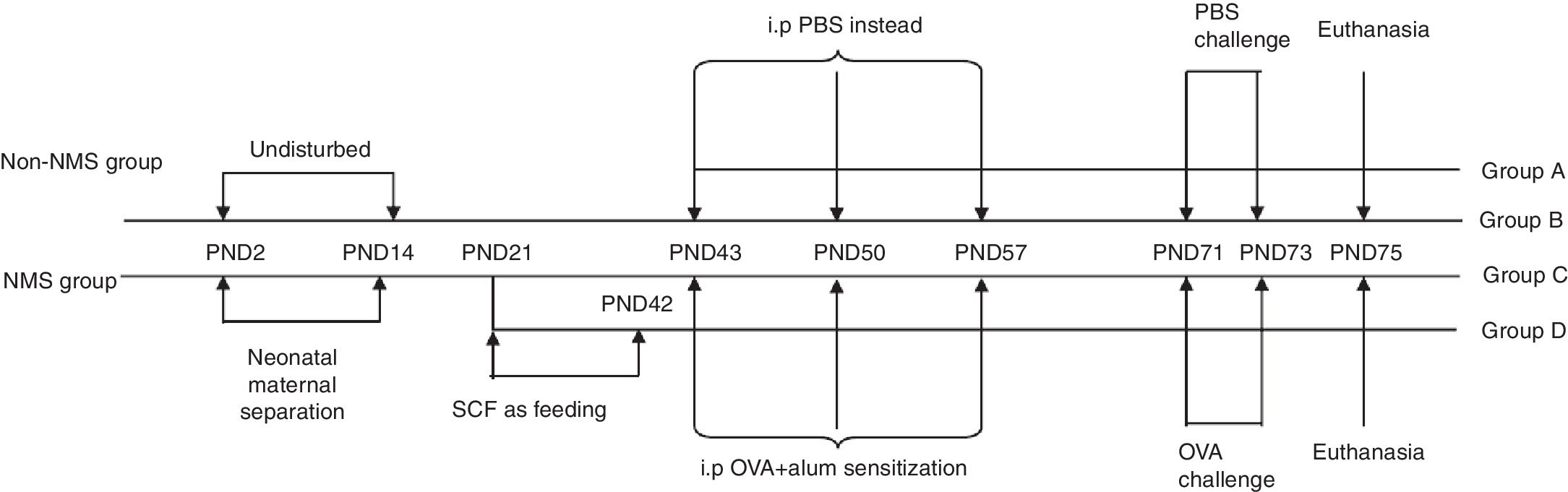
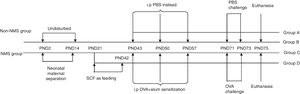
Asthma modelAsthma model was established as described by Oh et al.8 Briefly, mice in groups B, C and D were sensitized via intraperitoneal injection of 100μl PBS containing 20μg OVA and 2mg Al(OH)3 on PND43, 50 and 57, while mice in group A were given 100μl PBS instead. From PND71 to PND73, challenge-exposed litters were put in airtight cages and given aerosol inhalation of 10ml 10% OVA once a day, 1h per time, and mice in group A were given equivalent PBS. Forty-eight hours after the last allergen challenge, mice were euthanized and bronchoalveolar lavage fluid (BALF) and lung were collected. The complete experimental protocol is shown in schematic form (Fig. 1).
Experimental protocols. NMS litters were separated from their mothers for 3h daily from PND2 to PND14, while non-NMS ones were left undisturbed. All male mice were weaned on PND21. The change of feeding was between PND21 and PND42. Animals were later sensitized to OVA with intraperitoneal injections on PND43, 50 and 57, and were challenged with inhaled OVA dissolved in PBS on PND71, 72 and 73. All mice were sacrificed on PND75.
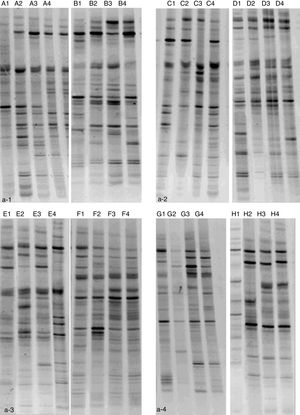
Feces of mice were collected on PND21, 28, 35 and 42. DNA was extracted from the collected fecal samples by traditional phenol chloroform method described by Chen and Huang9 All DNA samples were amplified with the primers (5′-ATT ACC GCG GCT GCT GG-3′) and (5′-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG GCC TAC GGG AGG CAG CAG-3′) which targeted to the V3 region of the 16s ribosomal DNA gene. Denaturing gradient gel electrophoresis (DGGE) was performed with a universal detection system run at 60°C, 220V for 10min and then 150V for 270min. Meanwhile, the number of SCFAs was measured using liquid chromatography.
Measurement of inflammatory cells and eosinophils (EOS) in BALFInflammatory cells and eosinophils (EOS) in BALF were detected using the methods described by Feng et al.10 First, each mouse was fastened in the supine position on the operation panel. After being disinfected, neck skin was separated from muscle to expose the trachea which was catheterized using a tube attached to a 22-gauge needle. Then the right lung was ligatured by surgical suture. Bronchoalveolar lavage was performed using 3× 0.8ml PBS. Fluid was transferred to a new EP tube and mixed. 50μl of the collected liquid was mixed with the same volume of leukocyte diluents. The mixture was detected for total inflammatory cells by the cell count plate, and the supernatant was then centrifuged at 4°C (3000/r/min) for 10min to collect the cell pellet for EOS counts.
Lung tissue pathological alterationAfter formaldehyde fixation, paraffin embedding, slicing, and hematoxylin–eosin (HE) staining, lung tissue was evaluated for pathological alteration under microscope.
The content of inflammatory factor in lung tissuesInterleukin (IL)-13, -25, -33 were measured in the lung tissues using commercially available enzyme-linked immunosorbent assay (ELISA) kits [IL-13 and IL-33: EK2132 and EK2332, Multi Sciences (Lianke) Biotech Co., Ltd., Hangzhou, China; IL-25: 85-88-7002-22, eBioscience, San Diego, USA] as specification. The lung tissues were kept frozen at −80°C until cytokine analysis.
Protein expression of Occludin and E-cadherin in lung tissueProtein expression of Occludin and E-cadherin in lung tissue was measured by Western blotting as below. Lung tissues were ground on ice, homogenized in pre-cooling lysis buffer (P0013B, Beyotime Biotechnology, Inc., Nanjing, China), and then centrifuged at 12,000rpm at 4°C for 20min. The supernatants were measured for protein concentration following the instruction of BCA Protein Assay Kit (KGP902, KeyGen Biotech Co., Ltd., Nanjing, China).
Protein samples were mixed with 5× loading buffer at 1:4, boiled at 100°C in metal plating baths for 10min, cooled at room temperature, and then boiled at 95°C for 10min. The supernatants were then obtained and electrophoresed on 8–16% SDS-polyacrylamide Tris–glycine gels. The gels were transferred onto nitrocellulose membranes, sealed with 5% skimmed milk, reacted respectively with rabbit anti-mouse Occludin (1:50,000–1:200,000) and E-cadherin (1:1000) antibodies at 4°C overnight, and then reacted with horseradish peroxidase labeled goat anti-rabbit IgG (1:10,000) at room temperature for 1h. After washing with TBST, ECL coloring solution was added to the membrane. Tublin was used as internal reference during the whole progress.
Statistical analysisStatistics were performed using the SPSS 21.0 software. The measurement data were expressed as mean±standard. Student's t-test was used to compare the difference in variables between two groups, while one-way ANOVA was performed for comparisons among more than two groups, and then Dunnett was used for further comparison. A p value <0.05 was considered statistically significant.
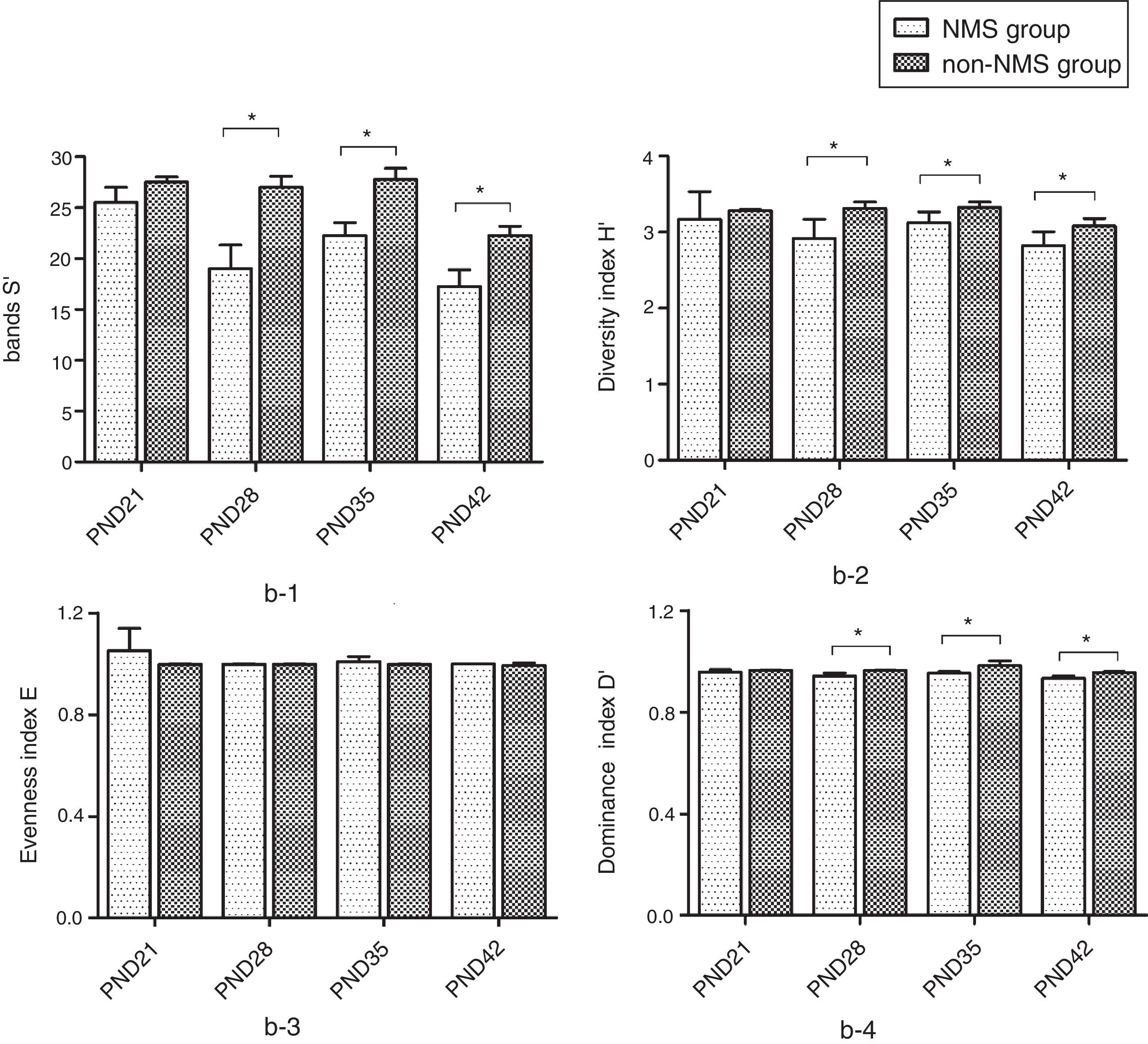
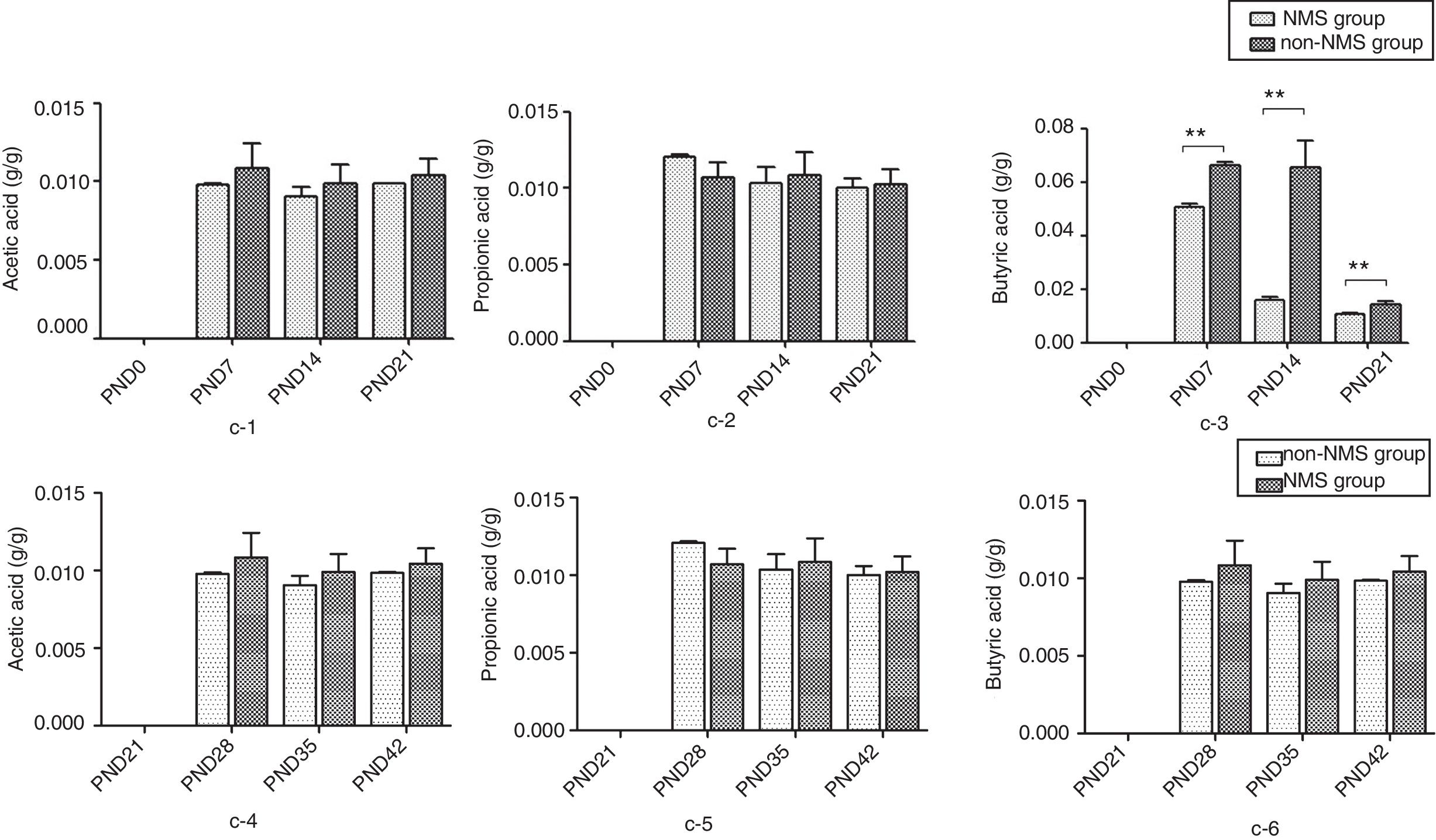
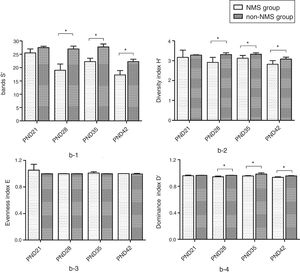
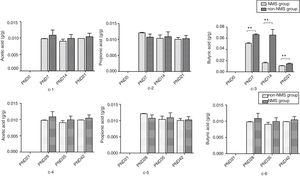
ResultsNMS effects on the intestinal microflora and SCFAsNMS influence on microflora of offspring was evaluated by DGGE. The results showed that the numbers of S′, diversity index H′ and dominance index D′, which were almost the same between NMS and non-NMS groups on PND0, were all significantly less in NMS group than those in non-NMS group on PND28, 35 and 42 (p<0.05) (Figs. 2 and 3). Liquid chromatography was conducted to measure the SCFA content in fetal samples, which showed that the level of butyric acid was significantly decreased at PND28, 35 and 42 (p<0.01) in the NMS group, respectively, than those in the non-NMS group, respectively, but not significantly changed of acetic acid and propionic acid between those two group at PND28, 35 and 42. In addition, mice in the NMS group had a significantly higher level of butyric acid after feed with SCFA during PND28, 35 and 42 (p<0.01) and a slightly but not statistically lower level of acetic acid than those in the non-NMS group at different time points (Fig. 4).
Diversity analysis of DGGE. b-1 represents the number of bands of intestinal flora in two groups. b-2 represents the number of diversity index of intestinal flora in two groups. b-3 represents the number of evenness index of intestinal flora in two groups. b-4 represents the number of dominance index of intestinal flora in two groups. The number of S′, diversity index H′ and dominance index D′ of the NMS group were significantly lower than those in the non-NMS group on PND28, 35 and 42, while the difference between the two groups was almost the same on PND21. *p<0.05.
The content of short-chain fatty acids. c-1 represents the content of acetic acid at PND21, 28, 35 and 42. c-2 represents the content of propionic acid at PND21, 28, 35 and 42. c-3 represents the content of butyric acid at PND21, 28, 35 and 42. c-4 represents the content of acetic acid in different periods. c-5 represents the content of propionic acid in different periods. c-6 represents the content of butyric acid in different periods. There was no difference in acetic acid and propionic acid levels in the two groups. The content of butyric acid in NMS group was significantly higher than that in group NMS, and the difference was statistically significant. **p<0.01.
An asthma model was established in order to explore the influence of NMS on the asthma. OVA-sensitized and OVA-challenged animals (groups B and C) exhibited the appearance of acute asthma, such as irritability, dyspnea, abdominal cramps and/or nodding breathing while group A did not present these signs.
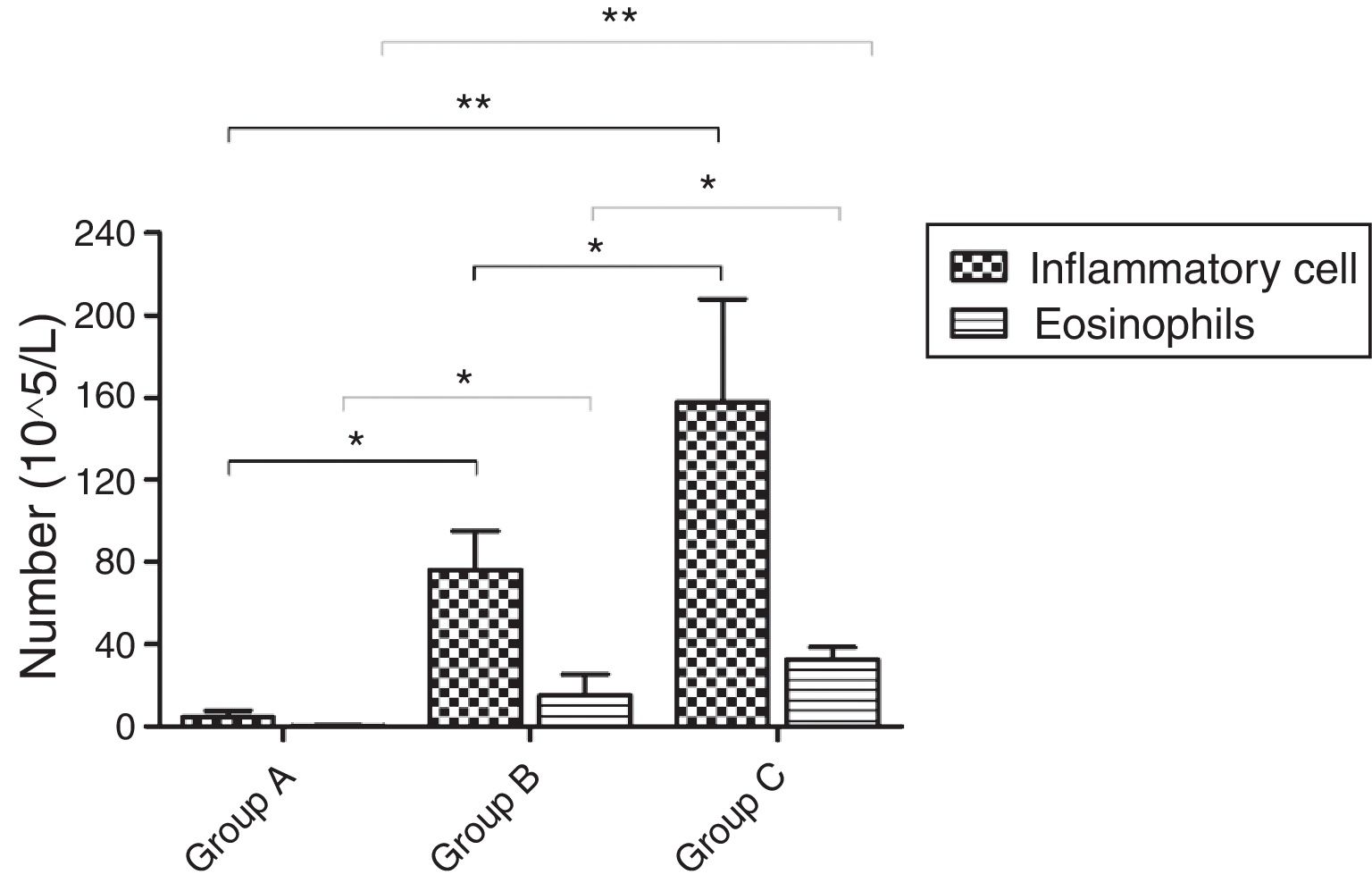
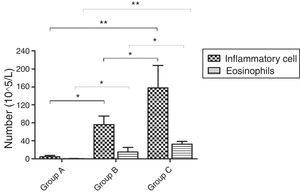
Inflammatory cellsOn PND75, a significantly increased number of inflammatory cells was found in BALF of OVA-sensitized and OVA-challenged male mice compared with PBS-sensitized and PBS-challenged animals (p=0.001). Further comparison showed that the number of total cells in group C was obviously higher than that in group B (p=0.023). The number of EOS increased in OVA-sensitized mice compared with PBS-sensitized ones (p<0.001). The same change in total EOS was also found between mice in group C and group B (p=0.005) (Fig. 5).
The number of inflammatory cells and EOS in BALF. d represents the number of inflammatory cells and EOS which were varied in the three groups. There was an increase in the mice of group C compared with these of group B while mice in group A had almost no inflammatory cells and EOS. *p<0.05 and **p<0.01.
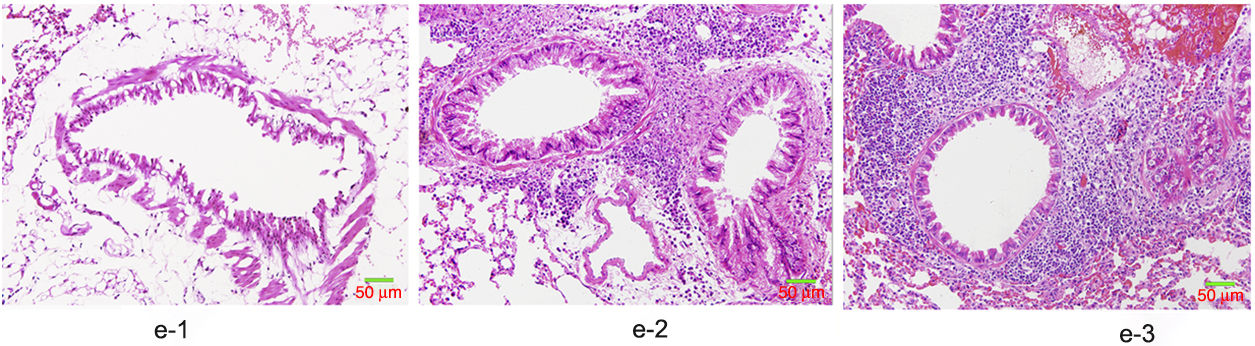
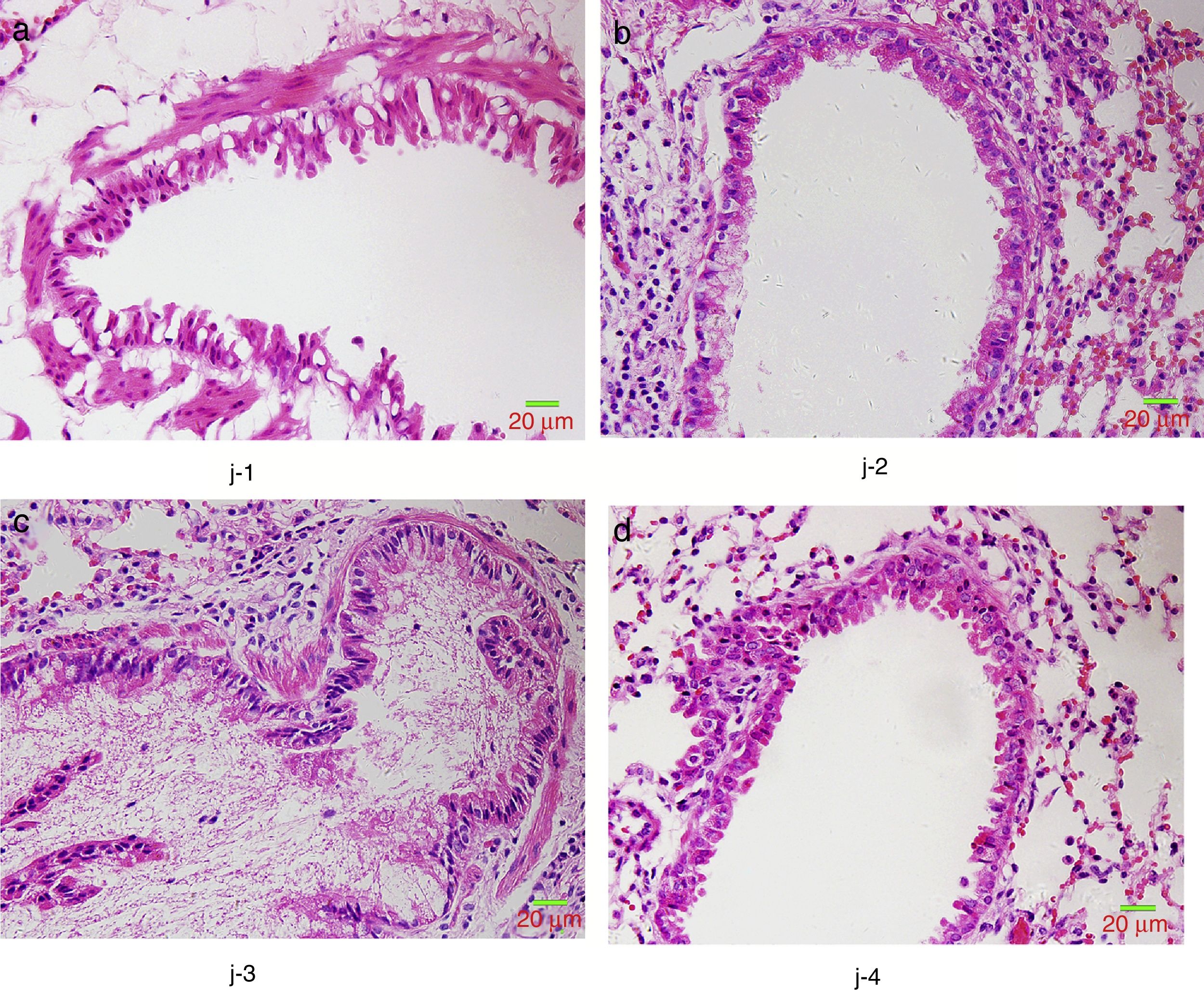
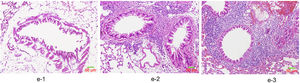
HE staining was conducted to observe the alveolar structure of mice, which showed clear and complete alveolar structure without emphysema, congestion or hemorrhage in mice of group A, but indicated interstitial infiltration in mice of OVA-sensitized and OVA-challenged group. The increased number of eosinophils and macrophages contributed to the increase of inflammatory cell total number. In addition, significant difference was shown in the pathological score among the three groups as group C>group B>group A (p<0.05) (Fig. 6).
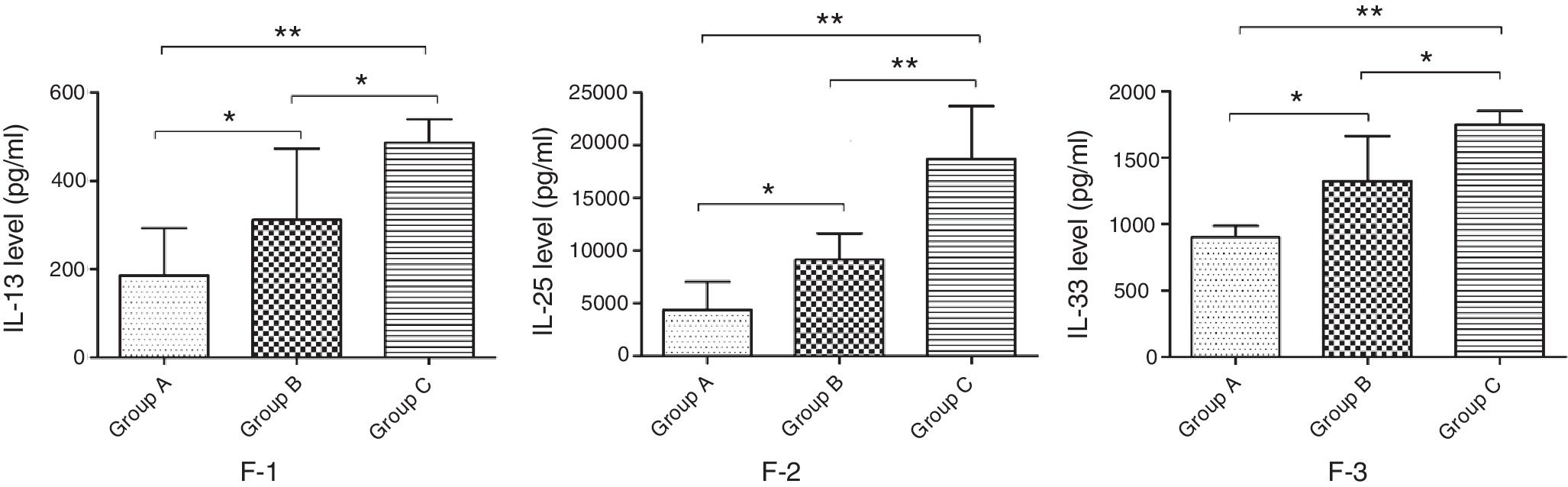
Changes of cytokine levels in lung tissueCytokines that could indicate the TH2 profile of the immune response in the lung were recently discovered and measured in lung tissue of group A, B and C animals to better understand the changes of inflammatory factors. The results showed that the concentration of IL-13, -25 and -33 in the lung tissue of mice in group B was significantly lower compared with mice in group C, but significantly higher than that in group A (p<0.05) (Fig. 7).
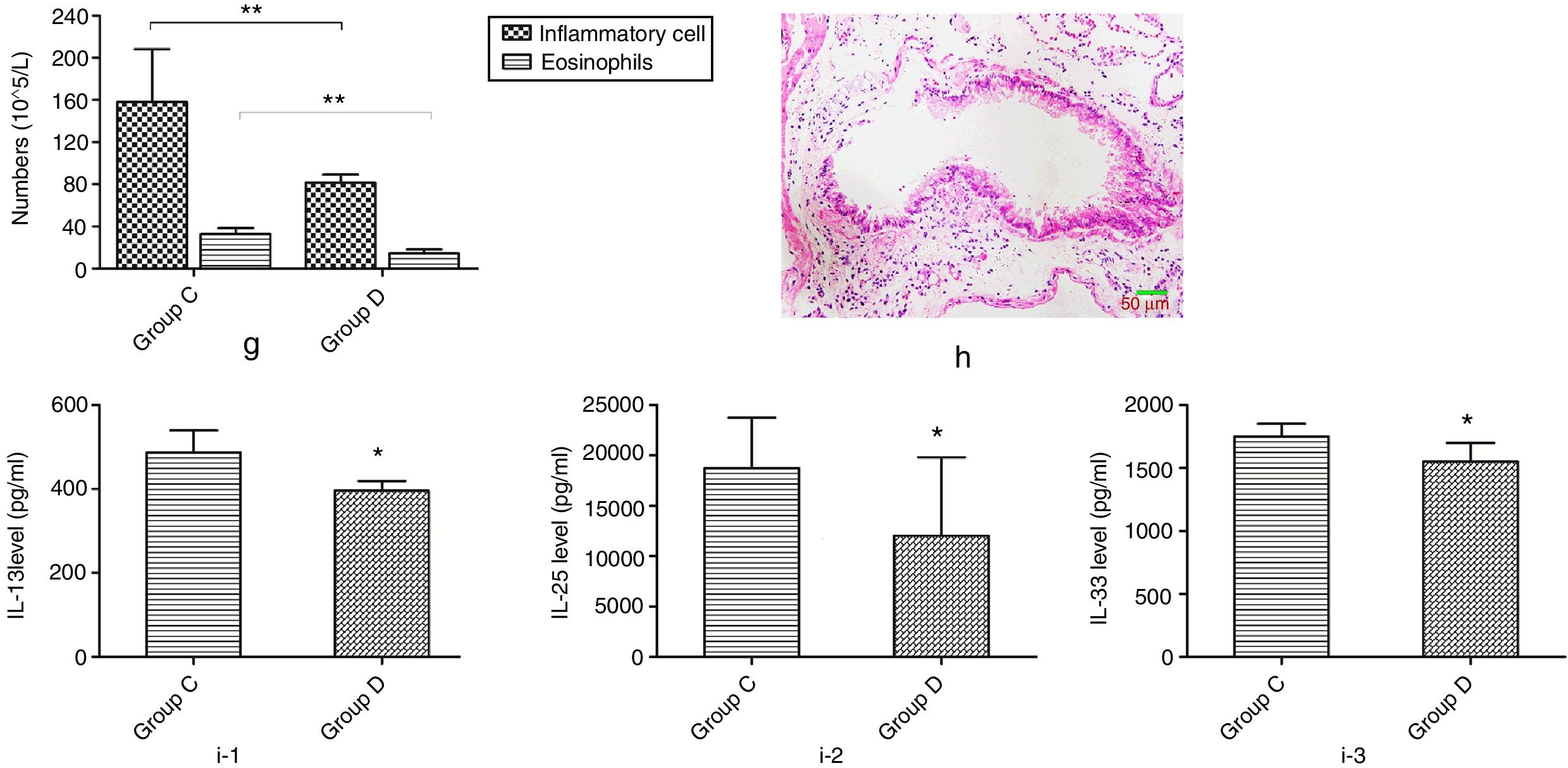
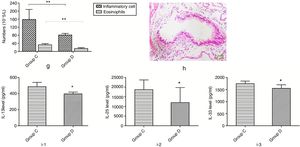
Effects of butyric acid on alleviated airway inflammation in NMS mice with asthmaMice in group D were additionally fed with butyric acid to explore the mechanism of the phenomenon described above. The number of inflammatory cells and eosinophils in mice of group D showed a significant decrease compared to that of group C. In additional, mice in group D also showed a significant lower pathological score and decreased level of IL-13, -25 and -33 than those in group C (Fig. 8).
Changes of inflammatory levels in asthmatic mice fed butyrate. g represents the numbers of the inflammatory cell and eosinophils. h represents the change of pathology in group D. i-1 represents the level of IL-13 in two groups. i-2 represents the level of IL-25 in two groups. i-3 represents the level of IL-33 in two groups. *p<0.05.
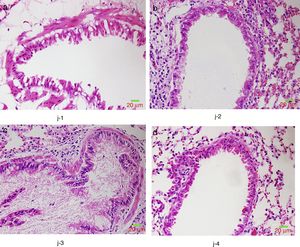
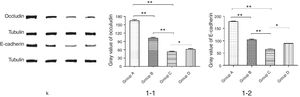
We further studied the effects of butyric acid on airway barrier by HE staining and connexin expression measurement. Mice in group D showed slightly decreased bronchial epithelium goblet cells and less sticky secretions in bronchial cavity than those in group C. The pathological changes in mice of group B were slight (Fig. 9). Furthermore, OVA-sensitized and -challenged mice showed a decline in the normalized expression of Occludin and E-cadherin in mice of group C compared to groups B and D (Fig. 10). No change was found, and Occludin and E-cadherin was normally expressed in mice of the control group A.
Intestinal microflora is a kind of normal microorganism in human body and it is estimated that a normal adult gut contains 1014 bacteria, approximately 500–1000 species.11 The human intestinal microbial colonization was traditionally thought to begin at birth, but a recent study suggests that colonization of neonatal intestinal flora is in the womb.12 The early stage of life is a critical period for the colonization and formation of intestinal flora. Back in 1999, foreign scholars used macaque as the object to establish a model of neonatal maternal separation, and found that the number of lactic acid bacteria decreased significantly in the three days after the separation and the integrity of the intestinal flora was thus destroyed.13 With the improvement of science and technology, Barouei et al.14 found that neonatals could increase the number of aerobic bacteria, anaerobic bacteria, Escherichia coli, Enterococcus and clostridium. The new study15 also found that the separation of mother and infant stress would change the composition of the intestinal flora. In the present study, we established an NMS mode, and confirmed that NMS would change the diversity of bacteria. This is consistent with the results of foreign studies.15,16
There are many types in human gut, including bacteroides, firmicutes, actinomycetes and so on, and they play an important role on the body, such as promoting digestion and absorption of vitamin synthesis and promoting metabolism and immunity against invading pathogens.17 One potential metabolism is SCFAs which have a profound influence in these effects. However, there has not been an experimental study on the changes of intestinal metabolites after the separation of mother and infant yet. Our study showed that NMS reduced the content of butyric acid in the gut. This result indicates the early-life stress effect on SCFAs in adult animals.
Effect of NMS on airway inflammation in asthmaEarly-life is a critical period for growth and development, and stress at this period will have a lasting impact on various organs including brain, lung, gastrointestinal tract and immune, which may not only lead to autism, depression, irritable bowel syndrome and other diseases,4,6,7 but also aggravate asthma and muscle pain.18–21 Scholars from Sweden have carried out a birth cohort study which showed that exposure to antibiotics within a week after birth increased the risk factors of asthma during school-age. It suggested that early life stress was associated with asthma.22 NMS is one of the early life stress events and it is worth exploring the relationship between NMS and asthma. At present, the NMS model has been widely used in psychology and intestinal behavior16,23,24; but rarely focuses on research about asthma. Kruschinski et al.25 studied the relationship between NMS and asthma by Fischer 344 rats, and found that NMS rats had an increased count of EOS and higher level of IL-13 and IgE than those in non-stress group. However, results from the study in 2010 showed that the number of leukocyte, eosinophils and the level of interferon gamma in mice after short maternal separation were lower than those in the control group.26
Asthma is a common global disease with incidence of up to 18%,27 and it has a huge impact on human life, work and study. Many kinds of cells, such as Th2 type cytokines and Th1 type cytokines, are involved in the development of asthma. It is known that IL-13, -25 and -33 are secreted by Th2 cells and play an important role in the process of allergic inflammation.28–30 In addition, in response to inflammatory cytokines, airway smooth muscle (ASM) cells may adopt a phenotype more suited to synthetic function with producing cytokines, chemokines, growth factors and cell adhesion molecules, which lead to contraction, spasm, hyperplasia and hypertrophy.31 The levels of IL-33 and IL-13 are increased in ASM and mast cells, and IL-33 can promote ASM contraction via upregulating mast cell-derived IL-13.32 Similarly, immunologic function of IL-25 in type 2 immunity is almost overlapped with IL-33, and IL-25 also regulates IL-13 to indirectly induce smooth muscle contraction.33 Thus, we speculated NMS may induce smooth muscle contraction via increasing inflammation and thus to aggravate asthma.
In our experiment, we found suffering from NMS stress increased the number of inflammatory cells, eosinophils in the BALF and the levels of IL-13, IL-33, IL-25 in the lung compared non-NMS group. The separation of mother and infant was found to be associated with aggravation of inflammation of airway in adult asthma from different angles. These results indicated that NMS could increase allergic airway inflammation in adult mice.
Potential mechanism of airway inflammation exacerbation induced by NMS in adultEffect of neonatal maternal separation on airway epithelial barrier in asthmaAsthma is a common chronic airway inflammatory disease, but its exact pathogenesis remains unclear. A shift in the imbalance between cytokines produced by Thl/Th2 cells is considered to be the main pathogenesis of asthma.34 In addition, the airway epithelial barrier also plays an important role in the development of asthma.35
The airway epithelial barrier is composed of airway epithelial surface fluid, airway epithelial cells and connections between epithelial cells. The airway epithelial surface liquid is the first line of defense against external harmful substances into the body; ciliary movement of ciliated cells can be discharged, harmful factor adhesion to mucus layer; and airway epithelium cell can also produce IL-1, IL-6, IL-8, IL-11, GM-CSF and TNF-α involved in host defense, airway inflammation, and airway remodeling35,36; epithelial barrier cell junctions include tight junction (TJ), adherens junction (AJ) and the gap junction, the former two are more important. TJ which can prevent the passage of hydrophilic solutes and Occludin plays an important role in the TJ barrier.37 AJ is composed of E-cadherin and deletion of E-cadherin can affect the TJ epithelial barrier function.38 Högman et al.39 found that hypertonic saline could increase the permeability of rat tracheal epithelial tight junction and made allergen easily through epithelial barrier and stimulated asthma. Because of the existence of the epithelial barrier in the airway, antigens cannot be easy to infringe on the subcutaneous tissue.
The NMS and asthma model were established to evaluate the airway epithelial barrier through pathological of airway epithelial changes. The expression of intercellular junction protein found around the epithelial cells of mice in asthmatic model group replaced by goblet cell, and the expression of Occludin and E-cadherin was lower than normal group. This showed that asthma would destroy the airway epithelial barrier, which was in accordance with other scholars’ research. We also found that NMS could aggravate asthma, and the mechanism might be related with the airway epithelial barrier.
Effect of SCFAs on airway epithelial barrier and airway inflammation in asthmaSCFAs are produced by the anaerobic bacteria in the colon. SCFAs in the human body mainly include acetic acid, propionic acid, butyric acid, valproic acid and so on. They have many important roles in many aspects, such as providing energy for host, providing nutrition to the epithelial cells and influencing the intestinal epithelial cells by effecting the growth, differentiation, transport and metabolism.40 In the study of the effects of diet on asthma, it was found that acetic acid could inhibit allergic airway disease by increasing the number and function of regulatory T cells.41 Our experiment showed that feeding SCFAs (butyric acid) in adult male mice reduced the airway inflammation, and the result was similar to that of other foreign scholars. The further study in our experiments found that SCFAs could alleviate the damage of asthmatic airway epithelial barrier, which indicated feeding SCFAs could improve the airway epithelial barrier and reduce airway inflammation.
In conclusion, we have shown that NMS induces significant changes in SCFAs and airway inflammation of adult asthma. The potential mechanism is that intestinal microflora diversity in early life is changed, and then causes the decline of butanoic acids, which leads to further damage on the airway epithelial barrier and finally results in asthma. However, with condition limitation, butanoic acids treatment and dose response curve analysis for asthma were not performed in the present study, and will be executed in our future study.
Conflict of interestAll authors declare that they have no conflict of interest to state.
This work was supported by Natural Science Foundation of Jiangsu Province (Project number: BK20151420) and National Natural Science Foundation of China (Project number: 81771628).