Allergy to cow's milk proteins has often been associated with dysfunction of the intestinal mucosa caused by chronic inflammation in infants. This study evaluated the protective effect of taurine on intestinal damage induced by beta-lactoglobulin (β-Lg) in Balb/c mice used as an animal model of allergy to cow's milk proteins.
MethodsBalb/c mice were treated with taurine administered orally by gavage (3mmol/kg/day) or intraperitoneally (100mg/kg/day) for two weeks, then sensitized intraperitoneally with β-Lg. The electrophysiological parameters: active ion transport of chloride (Short-circuit current: Isc) and the passive ion permeability (Conductance: G) were measured ex vivo in Ussing chamber by intestine challenge with β-Lg. Histological study was used to assess gut inflammation. Serum levels of TNF-α and IL-6 were measured. Serum IgG and IgE anti-β-Lg were determined by ELISA.
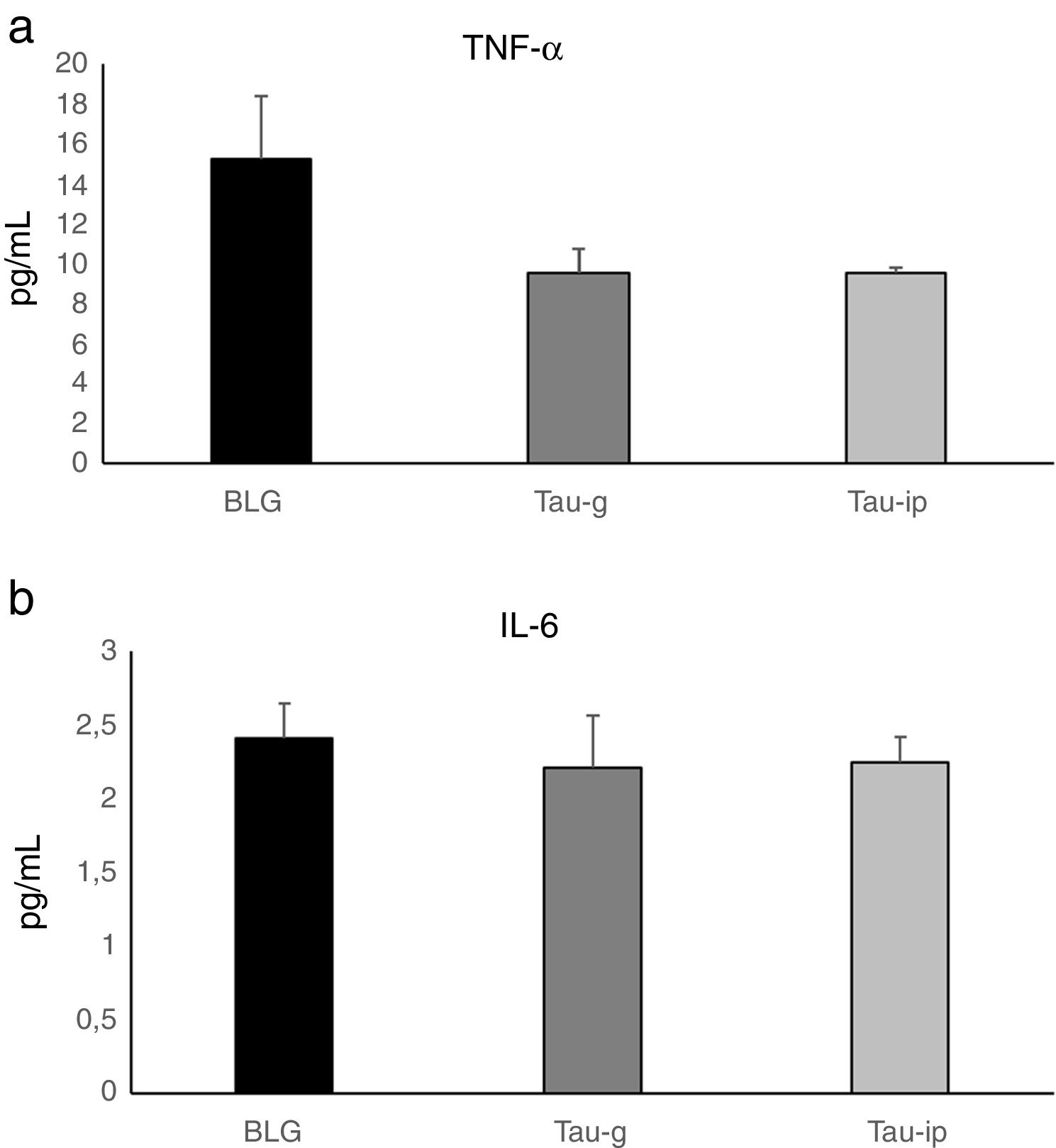
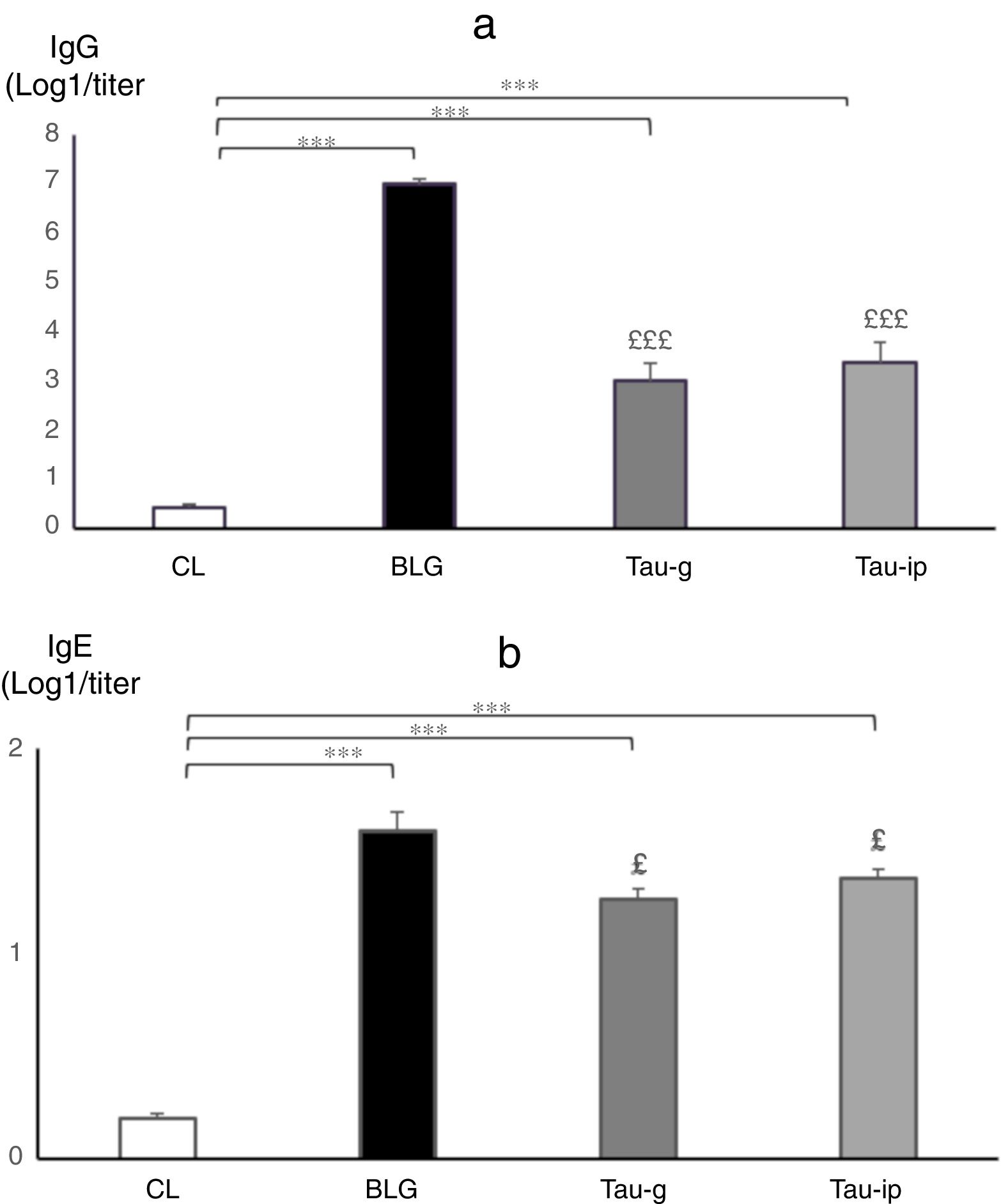
ResultsCompared with sensitized mice, β-Lg challenge of intestinal epithelium of taurine-pre-treated mice in Ussing chamber did not influence the intensity of Isc, nor produce any changes in the G, reflecting a reduction in the secretory response and epithelial permeability. Histological and morphometric analysis showed that taurine reduced the intestinal damage and limited intestine retraction caused by β-Lg sensitization. No statistically significant difference in the serum levels of TNF-α or IL-6 was found after oral or intraperitoneal administration of taurine. Treatment with taurine significantly decreased the IgG (p<0.001) and IgE anti β-Lg levels (p<0.05).
ConclusionsThese results have for the first time provided evidence that pre-treatment with taurine appears to prevent intestinal damage induced by β-Lg.
The prevalence of allergy to cow's milk proteins (ACMP) increases significantly; it affects 0.5–6% of the infant population.1 Its prevalence was approximately 2–3% in the infant population.2 The ACMP has often been associated with dysfunction of the intestinal mucosa caused by chronic inflammation in infants. The ACMP is characterized by an immediate type I hypersensitivity reaction3 involving IgE-dependent antibody responses. The allergic reaction is initiated by transporting the antigen through the intestinal epithelial cells resulting in a local hypersensitivity reaction. Thus, in allergic food reactions, an inflammatory response of the intestinal mucosa is associated with an imbalance of the intestinal barrier.4,5 Beta-Lactoglobulin (β-Lg), a major allergen of bovine milk, was shown to cause a severe intraepithelial lymphocytes infiltration, a partial villous atrophy and an important distortion of the epithelium architecture. These inflammatory responses were correlated with an increased intestinal permeability which resulted in epithelial barrier damage.6–8 Alterations of intestinal permeability appeared to be secondary to the absorption of cow's milk antigens.
In recent years, a number of studies have highlighted the positive effect of immunonutrition in the reduction of intestinal inflammation and have demonstrated that a diet based on sulfur amino acids possesses an interesting ability to modulate immune functions, particularly in the intestine.9,10 In this context, studies have shown the preventive and therapeutic role played by taurine (2-aminoethanesulfonic acid) in the treatment of several immune diseases, in particular certain intestinal disorders. Several studies indicated that taurine could be a good candidate capable of modulating the permeability of intestinal barriers10–12 by a direct action on the epithelium cells and an indirect action by suppression of the inflammatory reaction.13
This β-amino acid was already known for its therapeutic, anti-oxidant, anti-inflammatory, cytoprotective and immunomodulatory properties demonstrated in numerous studies14 and was proposed as a modulator of inflammation because of its ability to inhibit the production of pro-inflammatory mediators.15 In addition, the study by Sukhotnik et al.10 has clearly shown that the treatment of rats with taurine prevented intestinal damage of epithelial cells after intestinal ischemia-reperfusion. Moreover, several experimental data have demonstrated the relationship between taurine and the increase of organism immune function9 and emphasized its ability to prevent dextran sodium sulfate-induced colitis.12
However, little is known about its effect in type I allergy, particularly in ACMP. Therefore, the objective of this study is to evaluate the protective effect of taurine on intestinal damage induced by β-Lg, a major allergen of bovine milk in Balb/c mice used as an animal model of the ACMP.
Materials and methodsAnimals, taurine administration and sensitization protocolFifty-six female Balb/c mice (3–4 weeks old) were purchased from the Pasteur Institute of Algiers. The mice were maintained at a constant temperature of 21–24°C and exposed to a 12h/dark cycle light. Commercial standard food (Local Production of Bouzareah, Algiers) and water were given ad libitum for all the duration of the experiment. Mice were divided into four groups (14 mice in each group). The first group was untreated and represents the negative control (CL), the second group was sensitized with β-Lg (Sigma, France) and represents the positive control (BLG), the third and fourth groups were treated with taurine (Sigma-Aldrich, St. Louis, MO, USA) administered orally by gavage at a dose of (3mmol/kg/day=375mg/kg/day) (Tau-g)16 or intraperitoneally at a dose of (100mg/kg/day) (Tau-ip)17 respectively for 15 days, then sensitized with β-Lg. The oral and the intraperitoneal route were selected as the mode of administration in the clinical setting. Mice of the 2nd, 3rd and 4th groups were sensitized on days 0, 14, 21 and 28 by intraperitoneal injection (i.p.) of 10μg β-Lg adsorbed onto 2mg of alum [Al(OH)3] (Merck, France) in 0.1mL of phosphate-buffered saline (PBS).18
On day 50 of experimentation, the mice were anesthetized and segments of jejunum 10–20cm in length were excised for analysis in Ussing chamber. Small intestines were harvested for length and weight measurement. Blood was obtained from retro-orbital senous plexus and centrifuged.19 The sera obtained were collected for pro-inflammatory cytokines analysis. The method of blood sampling described in the protocol was chosen according to two main criteria: (1) The animal is under anesthesia and this should be less painful and stressful. (2) An effective method for collecting large volumes of blood and organs at a time. The experiments described in this study comply with the current Algerian legislation covering the protection of animals.
Ussing chamber experimentsTo study local anaphylactic response and epithelial permeability, the short-circuit current (Isc) and conductance (G) were measured ex vivo in Ussing chamber (Physiologic Instruments, San Diego, CA, USA) after intestine challenge with β-Lg following the established procedures.8–20 The jejunum was mounted in the Ussing chamber and challenged with 60μg/mL of β-Lg in the serosal side. This test determines the movement of electrolytes through the intestinal epithelium notably chloride (Cl−) expressed by Isc, while the conductance of tissue reflects the permeability of tight junctions. The electrophysiological parameters are measured every second throughout the experiment using Acquire & Analyze 2.3 software (Physiologic Instruments, San Diego, CA, USA).
In order to determine the nature of the secretory response, tissues of the β-Lg sensitized group were treated with a pharmacological substance, the compound 48/80at 5μg/mL added to the serosal side of the tissue. This non-immunological pharmaceutical agent is known to act rapidly on mast cell degranulation and to release histamine. The mechanism of mast cell degranulation induced by compound 48/80 involves direct activation of G protein.21
Histological analysisSegments of jejunum were processed for histological analysis. Tissues were fixed in 10% buffered formalin solution for routine processing. Six-micron-thick paraffin sections were stained with hematoxylin and eosin.22 The intestinal tissue damage (inflammatory infiltration and villous atrophy) was examined by light microscopy (Optica Axiom 5000, China). The measurements of the length of villi were taken using a micrometer optical microscope as a marker of inflammation.
Macroscopic symptoms: markers of inflammationTo evaluate intestinal inflammation, intestinal samples were placed on ice-cold plates, cleaned of fat and mesentery, and then blotted on filter paper. Each small intestine was weighed, the length and the disease activity index (DAI) were measured.23
Determination of TNF-α and IL-6 levelsThe effect of taurine on the pro-inflammatory cytokines was determined in vivo. Serum levels of Tumor Necrosis Factor alpha (TNF-α) and interleukin 6 (IL-6) were measured using Enzyme Linked Immunosorbent Assay (ELISA) kits (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer's instruction protocols.
Dosage of the IgG and IgE anti-β-Lg by ELISASpecific anti β-Lg antibodies were assayed in serum samples using an Enzyme-Linked Immunosorbent Assay (ELISA). More details of the ELISA procedure were reported by Zellal et al.18
Statistical analysisData are presented as mean±standard error of mean (SE). Comparisons between groups were performed using analysis of variance (ANOVA) or unpaired Student's t-test where appropriate. A p value of <0.05 was considered statistically significant.
Results and discussionThis work was conducted to evaluate the effect of taurine administration on the intestinal damage induced by β-Lg. In this study, Balb/c mice were used as a murine model of allergy, as described by our laboratory.24
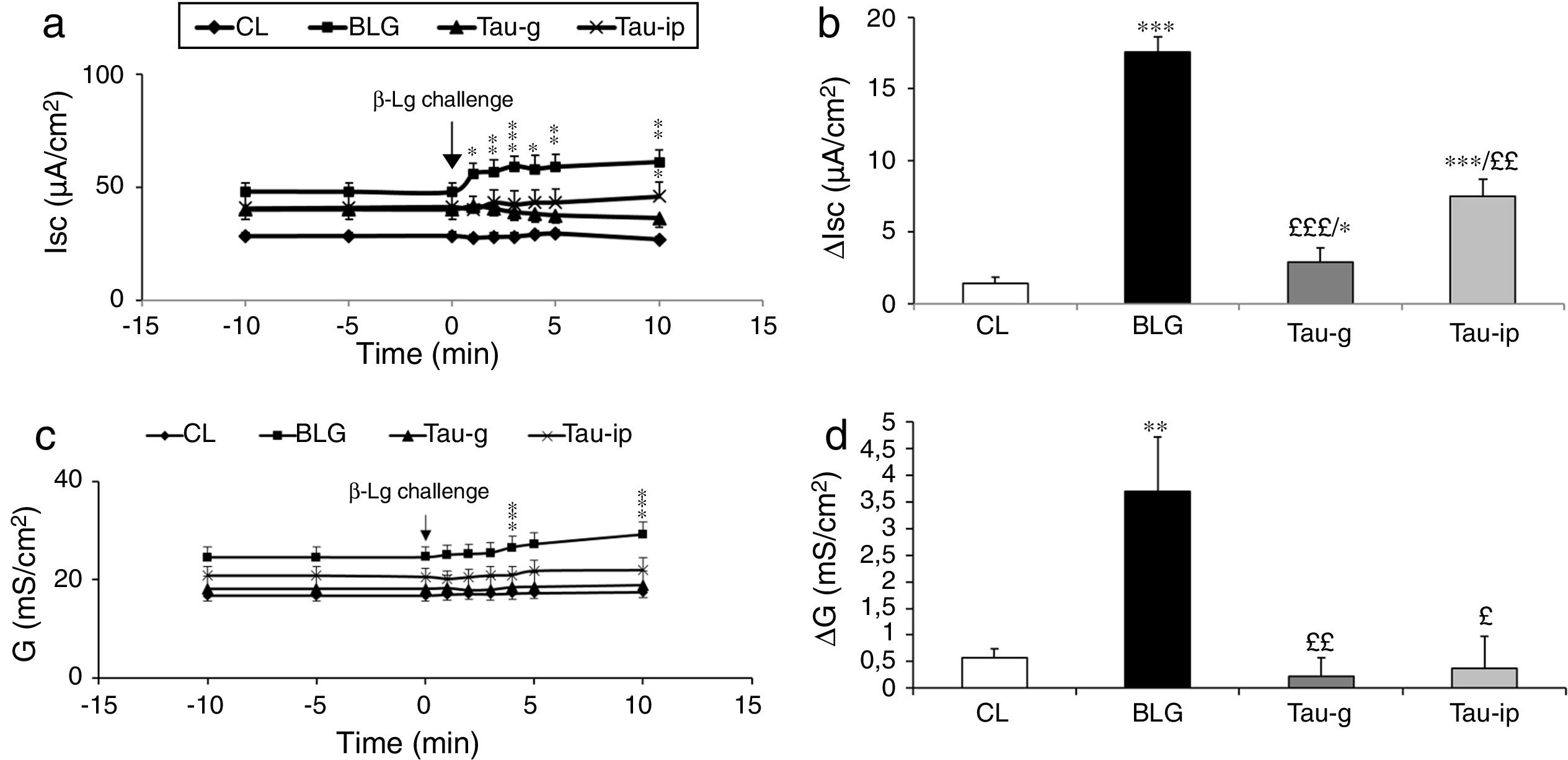
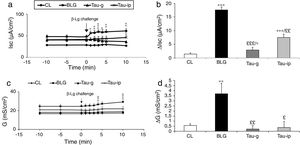
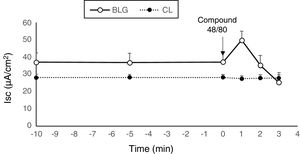
Compared with the CL group, the serosal β-Lg stimulation of jejunum from β-Lg-sensitized mice (BLG) in Ussing chamber was expressed by chloride ion secretion (Cl−), reflected by an important increase in Isc (ΔIsc=17.54±1.04μA/cm2) (p<0.001) (Fig. 1a and b). The increased Isc response was correlated with an increased tissue conductance (G) which is a measure of the integrity of tight junctions (ΔG=2.90±1.28mS/cm2) (p<0.001) (Fig. 1c and d) suggested an impairment of intercellular junctions of intestinal epithelium and the existence of a local anaphylactic response. Previous findings from our laboratory also showed the same results.8–25 Different mediators such as histamine, serotonin and prostaglandin released by activated mastocytes cells are at the origin of inflammatory reactions and symptoms in allergic reactions after contact with a specific food antigen.26,27 These substances are capable of inducing an active Cl− secretion in sensitized intestine.6–28
Effect of taurine administration on Isc (a), ΔIsc (b), G (c) and ΔG (d) values after β-Lg ex vivo challenge.
The increase in Isc, ΔIsc and G, ΔG is the difference between the peak value after β-Lg challenge and the baseline values. Values represent mean±SE (standard error) (n=8).
***p<0.001: BLG vs. CL; *p<0.05: Tau-g vs. CL; **p<0.01: Tau-ip vs. CL; £££p<0.001: Tau-ip vs. BLG; ££p<0.001: Tau-ip vs. BLG.
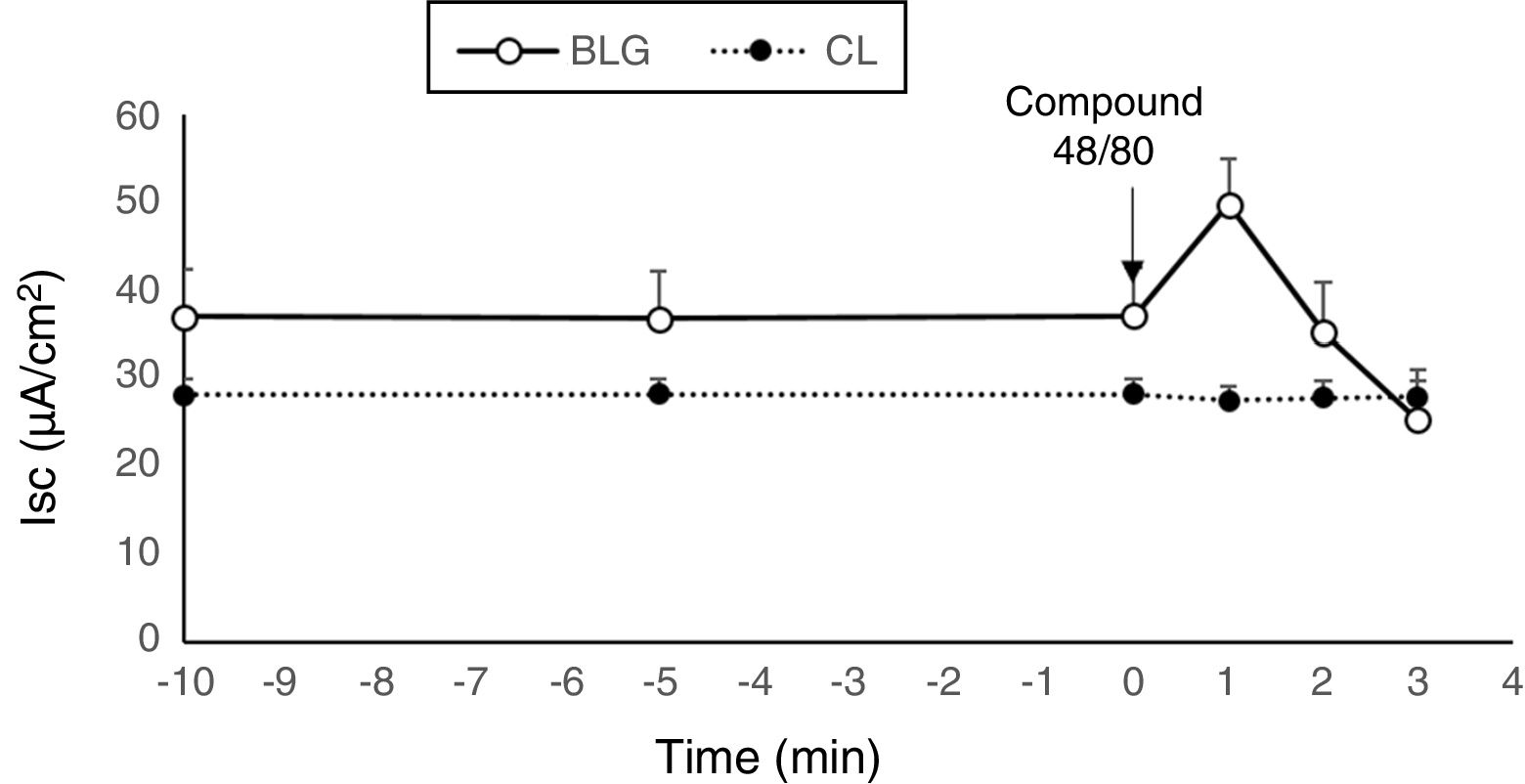
Tissues from β-Lg-sensitized mice challenged with compound 48/80, a mast cell degranulator, showed a marked increase in Isc (Fig. 2). This result indicates that the observed secretory response was probably due to a mast cell degranulation. Several studies demonstrated that compound 48/80 degranulates mast cells and that this effect is reversed by mast cell stabilizers.29 Compound 48/80, a potent endogenous histamine releaser, interacted with G-membrane proteins, activated phospholipase C and increased intracellular calcium stores, resulting in degranulation of mast cells.21
As shown in Fig. 1a and b, the serosal β-Lg challenge of the jejunum of taurine pretreated mice (Tau-g) and (Tau-ip) did not influence the intensity of Isc compared with the BLG group. This suggested a decrease of local anaphylactic reaction. In addition, β-Lg challenge of fragments of jejunum from taurine-pre-treated mice (Tau-g) and (Tau-ip) showed no increase in conductance (G) compared with the BLG group [(p<0.01) and (p<0.05), respectively] (Fig. 1c and d), suggesting a decrease in the permeability of the para-cellular pathway and a repair of the intestinal epithelial barrier function.
To our knowledge, this result is the first in the literature suggesting that taurine reduced epithelial permeability and local anaphylactic response after stimulation with β-Lg. The underlying mechanism may be linked to one crucial event, such as maintaining calcium homeostasis. Indeed, there is evidence supporting that taurine may play a major role in maintaining membrane stabilization, osmoregulation and calcium homeostasis. On the other hand and as explained above, histamine is capable of inducing an active Cl− secretion in sensitized-intestine.6–28 This substance is stored mainly in immune cells; its release is caused by the increase of [Ca2+] after antigen-antibody reaction via IgE receptor or chemical stimuli.30 Moreover, histamine receptors on intestinal epithelial cells caused an increase in cytosolic calcium and activation of protein kinase C.31,32 This caused a disruption of the junction proteins which can increase the para-cellular permeability.33 Endo et al.13 reported that amino acids can attenuate the increase of [Ca2+] in epithelial cells through two distinct processes: direct action on epithelial cells by influencing contraction of tight junctions and an indirect action by suppression of the inflammatory action due to the release of histamine. The likely hypothesis that taurine reduced epithelial permeability induced by β-Lg, preventing secretory response, is supported by these findings.
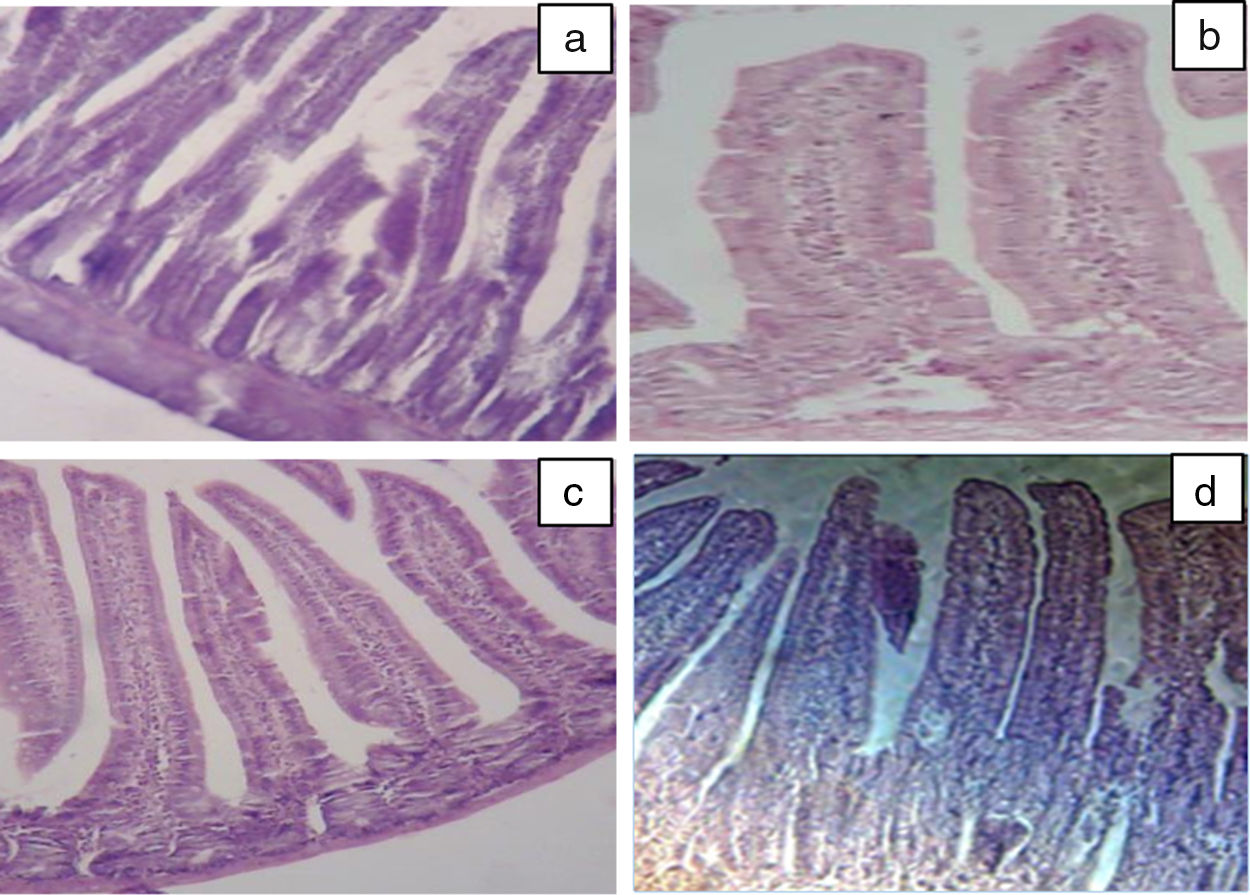
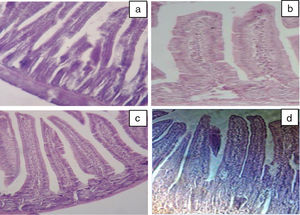
Our results of the histology study support the ex vivo results obtained in Ussing chamber. Histological examination of the intestine revealed the presence of a significant infiltration of inflammatory cells (intraepithelial lymphocytes) with a pseudo-stratified epithelium and villi atrophy (Fig. 3b) in BLG group. These results are in agreement with the study by Grar et al.8 The microscopic lesions caused by β-Lg were greatly attenuated by oral or intraperitoneal administration of taurine (Fig. 3c and d). However, it should be noted that oral administration of taurine has a more pronounced effect than its intraperitoneal administration.
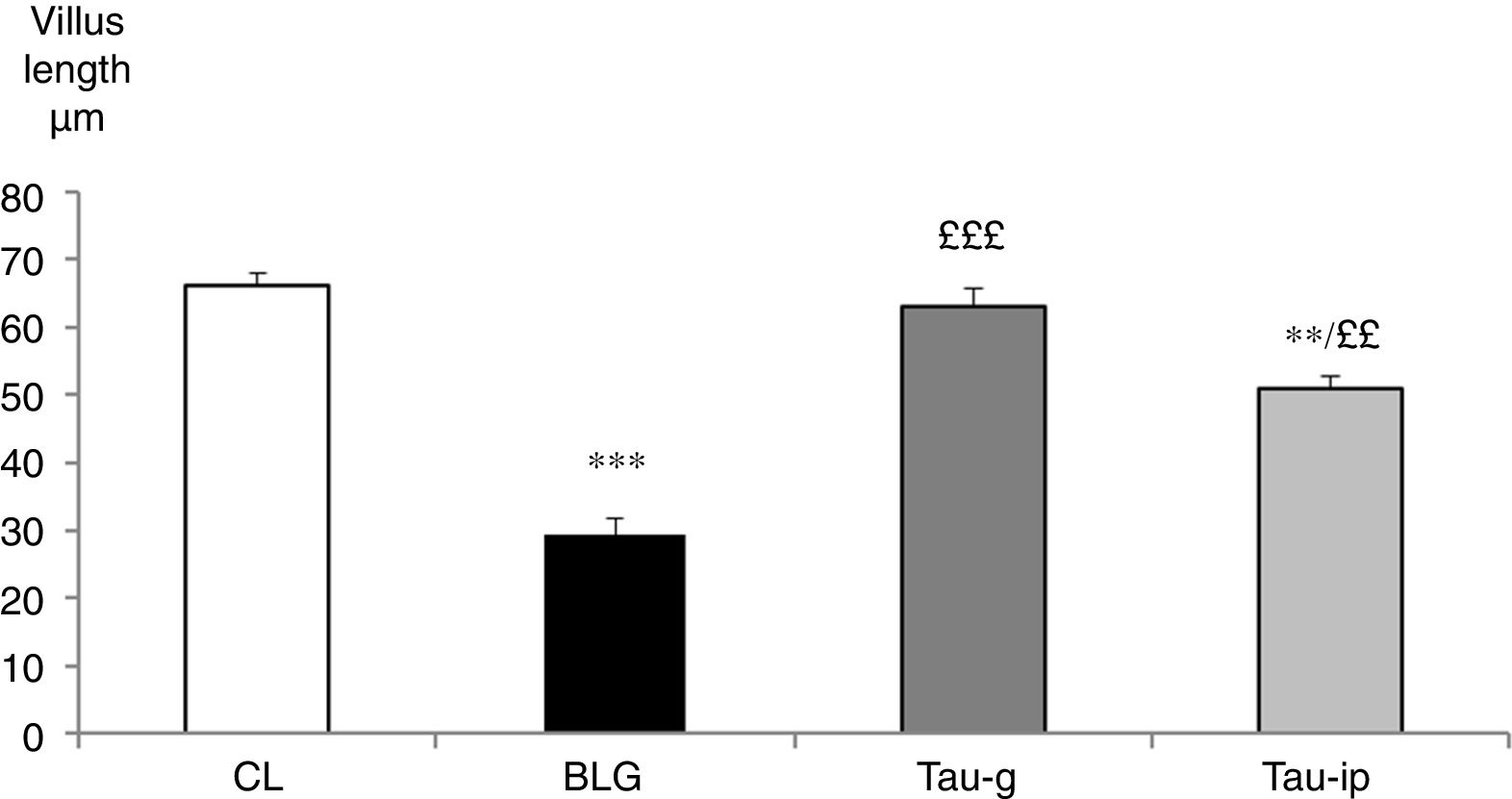
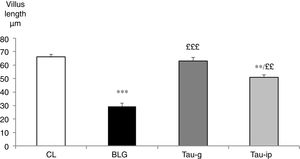
The villus length in the CL group was 66.06±2.04μm. It was very significantly reduced in the BLG group (29.37±2.42μm) (p<0.001) (Fig. 4). In the pre-treated groups (Tau-g) or (Tau-ip), taurine appears to inhibit the villus atrophy caused by β-Lg (63.11±2.71μm and 50.96±1.86μm, respectively) (Fig. 4). The surface of epithelium and villi were recovered. Thus, these data suggested that taurine may considerably protect the intestinal mucosa. This effect is probably related to its cytoprotective action. Similar observations indicating the protective effects of taurine on intestinal damage have been reported earlier by other studies.10,11
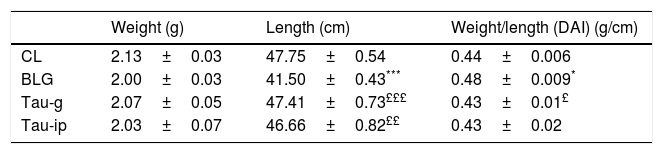
The disease activity index (DAI) is considered as an indicator of the severity of the inflammatory response.23 The morphometric analysis of the small intestine was performed (Table 1). Compared with CL group, a very significant decrease (p<0.001) in the length (p<0.001) was observed in BLG group. The intestinal weight/length (DAI) tended to increase significantly in this group (p<0.04). In contrast to the BLG group, the intestinal length of pretreated mice (Tau-g or Tau-ip) increased significantly (p<0.001 and p<0.01, respectively). DAI was similar in both (Tau-g) and (Tau-ip), suggesting that this β-amino acid can exert many beneficial effects on intestine of β-Lg-sensitized mice. This effect is probably due to its anti-inflammatory action. Indeed, taurine represents more than 50% of the pool of free amino acids in cells, which may indicate its importance in the modulation of the immune responses.34 Nevertheless, additional studies are needed to determine whether this effect is associated with the stimulation of epithelial growth factor or through decreased cellular apoptosis in the villus tips or through other mechanisms.
Effect of taurine administration on morphological measurements in the small intestine.
| Weight (g) | Length (cm) | Weight/length (DAI) (g/cm) | |
|---|---|---|---|
| CL | 2.13±0.03 | 47.75±0.54 | 0.44±0.006 |
| BLG | 2.00±0.03 | 41.50±0.43*** | 0.48±0.009* |
| Tau-g | 2.07±0.05 | 47.41±0.73£££ | 0.43±0.01£ |
| Tau-ip | 2.03±0.07 | 46.66±0.82££ | 0.43±0.02 |
Values represent mean±SE (standard error) (***p<0.001; ££p<0.01; £££p<0.001); (n=6).
*: BLG vs CL; £: Tau-g and Tau-ip vs. BLG.
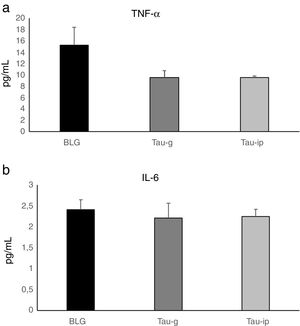
In this study, there was no statistically significant difference in the serum levels of TNF-α or IL-6 after oral or intraperitoneal administration of taurine compared with the BLG group (Fig. 5). These results agree well with those obtained by Ahn et al.35, in which taurine did not alter pro-inflammatory cytokine production on cerulein-induced pancreatitis and confirm that the anti-inflammatory effect of taurine on pro-inflammatory cytokines is a local effect. Indeed, Gurujeyalakshmi et al.36 and Zhao et al.12 have reported that the anti-inflammatory effect of taurine occurs locally in the inflamed tissues. These same researchers have suggested that the taurine could react with hypochlorous acid (HOCl), an extremely toxic oxidant, to produce taurine chloramines. This activity explains the anti-inflammatory properties of taurine, as its reaction with HOCl results in the generation of taurine chloramine, a more stable and less toxic anti-inflammatory mediator. This mediator has shown a powerful anti-inflammatory activity by depressing nuclear factor-κB (NF-κB) and down-regulating pro-inflammatory mediators such as TNF-α, interleukin (IL)-1β, IL-6 and IL-8.12–15
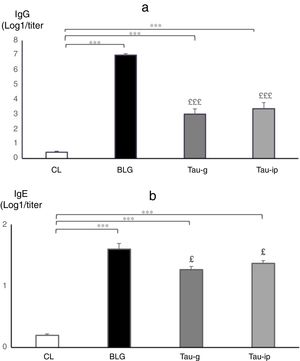
A significant increase in IgG (p<0.001) (Fig. 6a) and IgE anti β-Lg titers (p<0.001) (Fig. 6b) was observed in the BLG group compared with the CL group. The intraperitoneal sensitization using alum as an adjuvant induced the production of specific IgE in the serum of these animals37 which reflected an IgE dependent immune response.38 Interestingly, in the pre-treated groups (Tau-g) or (Tau-ip), taurine appears to cause a significant decrease in IgG (p<0.001) (Fig. 6a) and IgE anti β-Lg levels (p<0.05) (Fig. 6b) compared to the BLG group (Fig. 6a and b). Numerous studies have shown that IgE production in response to a particular allergen was associated with some MHC class II alleles (major histocompatibility complex). This initiates a Th2 response (type I allergic reaction)39 as well as of other inflammatory processes. Taurine plays an important role in the immune system as a key modulator of granulocyte and macrophage reactivity through its antioxidant capacity in addition to its crucial role on surface markers of antigen presenting cells (APC) particularly class II MHC antigens.40 The in vitro study by Marcinkiewicz et al.41 showed that taurine derivative (Taurine chloramine) exerts an inhibitory effect on T-cell and APC in the presence of ovalbumin, suggesting an indirect role of taurine by modulating the inductive phase of a specific adaptive immune response.
ConclusionsIn the present study, we evaluated the preventive effect of taurine administrated orally or intraperitoneally on intestinal damage induced by β-Lg in a murine model of allergy. The variation of electrophysiological parameters (Isc) and (G) measured ex vivo in the Ussing chamber were significantly inhibited by the administration of taurine, reflecting a reduction in the secretory response and epithelial permeability. Histological and morphometric analysis showed that taurine reduced the intestinal damage and limited the intestinal retraction caused by β-Lg sensitization. Pre-treatment with taurine had no effect on the serum levels of TNF-α and IL-6 but significantly reduced the serum anti-β-Lg IgG and IgE. It should be noted that the effects of taurine were only local, and the best route of administration is the oral route at a dose of 3mmol/kg/day. The present study suggested that pre-treatment with taurine may have an important implication in the attenuation or the prevention of intestinal inflammation during the ACMP. However, further studies are necessary to improve its preventive and therapeutic effectiveness.
Compliance with ethical standardsThe experiments described in this study comply with the current Algerian legislation covering the protection of animals.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflicts of interest to declare.
This research was supported by the Directorate General for Scientific Research and Technological Development (DGRSDT, MESRS, Algeria). The authors would like to thank Mr Thierno Aliou Diallo for accepting to read the present article.