Sublingual immunotherapy (SLIT) has been widely used for the treatment of allergic respiratory diseases, but many problems remain unsolved. Currently available data suggest that SLIT is very effective in children and adults with IgE-mediated respiratory diseases. Most allergists in China generally believe that SLIT is suitable for allergic rhinitis and asthma due to its safety and tolerability. SLIT for three years is suitable for patients to acquire stable therapeutic effects, and the efficacy of single-allergen SLIT for polysensitized patients has also been confirmed. Nevertheless, there are still several factors restricting its application in China, such as the uncertainty of its long-term effects and the prevention of new sensitizations onset, the risk of asthma attacks, the low public awareness of SLIT and poor compliance by patients. This is a narrative review of current evidence on SLIT coming from China.
Allergic rhinitis (AR) and asthma are common allergic disorders that greatly influence the quality of life of patients and can engender heavy economic losses.1 The prevalence of AR in the 18 major cities of China is appropriately 17.6% and has tended to increase gradually.2 According to a cross-sectional survey in eight metropolitan in China, the prevalence of asthma in children aged 6–13 years is 3.3%.3
Nowadays, Allergen immunotherapy (AIT) is strongly recommended for the treatment of AR and asthma by reducing respiratory symptoms, lowering the use of medication, and improving the quality of life.4 Moreover, it is considered to be a disease-modifying therapy which is capable of preventing the onset of new allergen sensitizations and the progression of respiratory allergies.5 Subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) are the most common approaches for AIT. In the past two decades, there has been a substantial increase in the use of SLIT for IgE-mediated respiratory allergies due to its safety and well-tolerance.6 SLIT can prevent allergen sensitization and inhibit allergic inflammation by inducing the generation and activation of Treg and Breg cells, modulating allergen-specific IgE and IgG-mediated responses, as well as inhibiting mast cell and basophil degranulation.7
The only standardized Dermatophagoides farinae (Der f) drops (Wolwo Pharma, Zhejiang, China), officially approved by the China Food and Drug Administration in 2006, have been widely used for clinical application in more than 800 tertiary and secondary hospitals of nearly 30 provinces and regions in China, but not in Tibet, Inner Mongolia, Hong Kong and Taiwan. The biologically standardized Der f drops are labeled according to the concentration of total protein in μg/mL (Number 1, 1μg/mL; Number 2, 10μg/mL; Number 3, 100μg/mL; Number 4, 333μg/mL; Number 5, 1000μg/mL) determined by the bicinchoninic acid protein assay.
Efficacy of SLIT for mite-related respiratory allergyMechanisms of SLITThe Der f drops for SLIT have been used in China for more than 10 years, and the clinical efficacy has been widely confirmed.8–10 SLIT has various effects on the immune system with multiple mechanisms. Firstly, SLIT corrects the immune biases by shifting the Th2 secretory profile to a Th1 cytokine pattern. House dust mite (HDM)-sensitized children with AR receiving SLIT showed a shift from Th2 to Th1 inflammation and decline in production of Th2 cytokines (IL-4, IL-5, IL-13) in peripheral blood mononuclear cells.11 Secondly, SLIT persistently decreases allergen-specific IgE synthesis and promotes the production of IgG4 blocking antibodies. In a multicenter, controlled, randomized, open-label study, after SLIT for 12 months, the specific IgG4 significantly increased compared to the control group, while the IgE/IgG4 ratio significantly decreased in contrast with the baseline.12 Thirdly, the generation and activation of Treg cells induces the allergen tolerance. Tian et al.9 reported that, after 12 weeks of SLIT with Der f drops, the numbers of cluster of differentiation CD4+CD25+Treg cells markedly increased in asthma patients allergic to HDM. No biomarkers were reliably recommended for the selection of individual patients in routine practice for SLIT, nor for the monitoring of the response to treatment. However, rapid advances in the molecular diagnosis would provide an exciting opportunity to implement the prediction of response to SLIT.
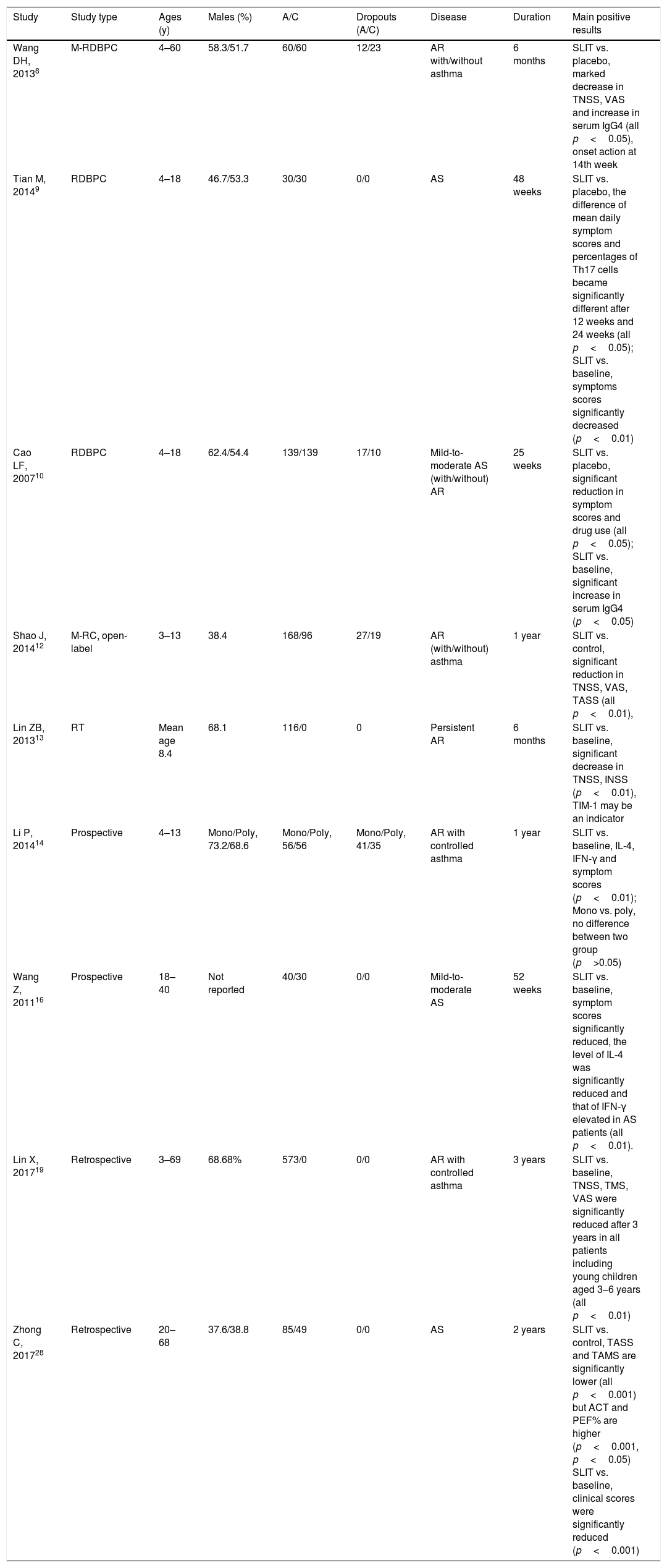
Efficacy of SLIT for allergic rhinitis and asthmaSymptom and medication scores (SMS) are widely recommended for the measure of efficacy in randomized controlled trials of AIT.36 The rhinoconjunctivitis quality of life questionnaire (RQLQ) and visual analog scale (VAS) may also be helpful. Likewise, In China, symptom scores and medication scores are most frequently used for evaluating the clinical effects. The EAACI Guidelines on AIT published in 2017 indicated that SLIT with HDM tablets was indicated for AR for short-term benefit and long-term benefit, while SLIT with aqueous solutions may not be recommended for perennial AR.4 However, Several clinical studies in China, including three randomized, double-blind, placebo-controlled (RDBPC) trials, two randomized controlled trials and a few prospective trials confirmed the efficacy and safety of SLIT with Der f drops in patients with AR8,10,12–15,27 and allergic asthma9,10,12,14–16,28 induced by HDM (Table 1). This inconsistency may be mainly caused by the following reason that the quantity of research related to SLIT with aqueous solutions in western countries is comparatively small. Wang DH8 prospectively confirmed that SLIT with a mixture of Der f and Dermatophagoides pteronyssinus (Der p) extract is effective and safe for patients aged four to 60 years old with HDM-induced AR. In another RDBPC clinical research involving four to 18 years old patients with moderate allergic asthma by Tian et al.,9 the symptom scores of asthma significantly reduced compared with placebo after 48 weeks of SLIT with Der f drops. Zhong et al.28 proved that SLIT with Der f drops plus pharmacotherapy was more effective than routine drug treatment in adult patients with allergic asthma. But, only few research gave high quality evidence and further related studies were needed.
Characteristics of clinical studies assessing the effects of SLIT on house dust mite respiratory allergy.
| Study | Study type | Ages (y) | Males (%) | A/C | Dropouts (A/C) | Disease | Duration | Main positive results |
|---|---|---|---|---|---|---|---|---|
| Wang DH, 20138 | M-RDBPC | 4–60 | 58.3/51.7 | 60/60 | 12/23 | AR with/without asthma | 6 months | SLIT vs. placebo, marked decrease in TNSS, VAS and increase in serum IgG4 (all p<0.05), onset action at 14th week |
| Tian M, 20149 | RDBPC | 4–18 | 46.7/53.3 | 30/30 | 0/0 | AS | 48 weeks | SLIT vs. placebo, the difference of mean daily symptom scores and percentages of Th17 cells became significantly different after 12 weeks and 24 weeks (all p<0.05); SLIT vs. baseline, symptoms scores significantly decreased (p<0.01) |
| Cao LF, 200710 | RDBPC | 4–18 | 62.4/54.4 | 139/139 | 17/10 | Mild-to-moderate AS (with/without) AR | 25 weeks | SLIT vs. placebo, significant reduction in symptom scores and drug use (all p<0.05); SLIT vs. baseline, significant increase in serum IgG4 (p<0.05) |
| Shao J, 201412 | M-RC, open-label | 3–13 | 38.4 | 168/96 | 27/19 | AR (with/without) asthma | 1 year | SLIT vs. control, significant reduction in TNSS, VAS, TASS (all p<0.01), |
| Lin ZB, 201313 | RT | Mean age 8.4 | 68.1 | 116/0 | 0 | Persistent AR | 6 months | SLIT vs. baseline, significant decrease in TNSS, INSS (p<0.01), TIM-1 may be an indicator |
| Li P, 201414 | Prospective | 4–13 | Mono/Poly, 73.2/68.6 | Mono/Poly, 56/56 | Mono/Poly, 41/35 | AR with controlled asthma | 1 year | SLIT vs. baseline, IL-4, IFN-γ and symptom scores (p<0.01); Mono vs. poly, no difference between two group (p>0.05) |
| Wang Z, 201116 | Prospective | 18–40 | Not reported | 40/30 | 0/0 | Mild-to-moderate AS | 52 weeks | SLIT vs. baseline, symptom scores significantly reduced, the level of IL-4 was significantly reduced and that of IFN-γ elevated in AS patients (all p<0.01). |
| Lin X, 201719 | Retrospective | 3–69 | 68.68% | 573/0 | 0/0 | AR with controlled asthma | 3 years | SLIT vs. baseline, TNSS, TMS, VAS were significantly reduced after 3 years in all patients including young children aged 3–6 years (all p<0.01) |
| Zhong C, 201728 | Retrospective | 20–68 | 37.6/38.8 | 85/49 | 0/0 | AS | 2 years | SLIT vs. control, TASS and TAMS are significantly lower (all p<0.001) but ACT and PEF% are higher (p<0.001, p<0.05) SLIT vs. baseline, clinical scores were significantly reduced (p<0.001) |
A/C: active/control; AD: atopic dermatitis; AR: allergic rhinitis; AS: allergic asthma; CVA: cough variant asthma; IFN: interferon; IL: interleukin; INSS: individual nasal symptom score; M: multicenter; Mono: monosensitized; M-RC: multicenter-randomized controlled; RCT: randomized controlled trial; PEF: peak expiratory flow; Poly: polysensitized; RDBPC: randomized, double-blind, placebo controlled; SLIT: sublingual immunotherapy; TASS: total asthma symptom scores; TIM: T-cell immunoglobulin and mucin; TNSS: total nasal symptom scores; VAS: visual analog scales.
Age does not appear to be a limitation for SLIT, and since the immune system could be modulated from infancy to old age, children were recommended to receive SLIT at an early age, when disease progression may be influenced more readily.17 Besides, considering the poor compliance in young children, SLIT was suggested to be initiated after the age of four years old.17 A meta-analysis from Italy demonstrated that SLIT was effective in patients aged 3–18 years with AR.18 Similarly, in China, several studies focused on the application of SLIT in young children. Shao et al.12 revealed that one-year SLIT with Der f drops remarkably improved the clinical symptoms and reduced drug use in children with AR aged 3–13 years. There was no difference in efficacy between children aged 3–5 years old and 6–13 years old. Lin et al.,19 confirmed the efficacy and safety of SLIT with Der f drops in 573 children and adult patients with HDM-induced AR, including the very young children less than four years old. In the study conducted by Li et al.,14 symptom scores and medication scores in those aged 4–13 years with allergic asthma significantly decreased after one year of SLIT. Thus, recent studies suggest that SLIT was also effective in young children.
Effects of single- allergen SLIT for polysensitized patientsAccording to epidemiologic and clinical studies, polysensitization is more prevalent than monosensitization in the general population.20 Recently, a multicenter study assessing the prevalence of sensitization in patients with respiratory allergies revealed that more than 90% of individuals in China were sensitized to two or more allergens.21 SLIT with a single allergen was also effective to polysensitized patients, probably because of the cross-reactions among different allergens and suppression of immune responses by regulatory cytokines (IL-10 and transforming growth factor-β) secreted by activated Treg cells.22,23
The EAACI Guidelines on AIT also emphasized the viewpoint that either a single allergen or a mixture of well-documented homologous allergens from the same biological family were recommended for patients with AR who were allergic to HDM.4 Similarly, several studies on the efficacy in monosensitized and polysensitized children with HDM-induced allergic diseases were conducted in China. Li et al.14 indicated a significant improvement in clinical parameters in monosensitized and polysensitized children with respiratory allergy after SLIT, with no significant difference between them. These results were in accordance with the guidelines and previous studies.
Other benefits of SLIT for respiratory allergyLong-term efficacy of SLITSLIT induces immune tolerance to allergens and sustains long-term efficacy after discontinuation.24,25 The therapeutic effects of SLIT remained two or more years after SLIT withdrawal. Since SLIT was applied for just one decade in China, the long-lasting benefits of SLIT were rarely reported. Therefore, further studies were needed. Lin Z et al.26 indicated that patients with HDM-induced AR achieved one-year long-lasting clinical benefits from SLIT.
Preventive effects of SLITIt is widely believed that AIT may prevent the development of new sensitizations and asthma.7 But, in the latest EAACI Guidelines published, AIT seemingly cannot be currently recommended for the prevention of new sensitizations in children and adults with AR and/or asthma, due to the lack of strong evidence.1 In China, only one study performed by Shao et al.12 suggested SLIT may prevent the onset of new sensitizations. In this study, the prevalence of new sensitization was significantly lower in children who received SLIT for one year than that in control group (3.55% vs. 27.27%). This study lasted for merely one year. Similarly, more RDBPC trials are required.
Safety of SLITSLIT is widely considered to be a safe alternative to conventional SCIT, with fewer and less severe adverse events (AEs)7. The frequent AEs were in the oral mucosa (itching and swelling) and digestive system. Moderate/severe events occurred only in a few cases which need medical intervention. Cox et al.29 reviewed 66 clinical studies on SLIT, in which 1,181,654 doses were administered to 4378 patients and no serious life-threatening reactions were reported. A survey from Italy confirmed the safety of SLIT in 126 children below five years old who received a total of 3900 doses for two years.30 So far, no serious AEs of SLIT have been reported in China. Shao et al.12 assessed the AEs of SLIT in children aged three to 13 years old with respiratory allergies. Fifty-four AEs from 39 children were reported, with no severe systemic AEs involved. Most of the AEs were grade 1 and relieved within one week with or without medication. Lin et al.19 evaluated the safety and good-tolerance of SLIT in adults and the young children with AR. No severe systemic AEs were reported. These studies confirmed that SLIT was safe and reliable in children and adults. It is noteworthy that patients and guardians should be told how to recognize AEs because SLIT was usually administered at home without direct medical supervision.
Patient compliance of SLITThe compliance of patients is a key factor for the success of SLIT on account of its long duration. One study from Italy demonstrated that after 18 weeks of SLIT, the prevalence of compliance of the dust-mite group and pollen group was 96.8% and 97.6%, respectively, and that of total compliance was 97.3%.31 Several findings suggest that compliance with SLIT is good, despite therapy being self-managed at home.
Some studies from China focused on patient adherence and analyzed the termination reasons as well. Wang et al.32 demonstrated that the dropout rate in the first year of SLIT among AR patients was 54%. The main impact factors included, the inability to reach patients, ineffectiveness, and the long course of treatment. Therefore, providing sufficient education about SLIT and timely follow-up via telephone may improve the compliance of patients.
Concluding remarksUp to now, SLIT has been applied for more than 30 years and is recognized as a much safer approach than SCIT in many practice guidelines 33 and 34. In China, SLIT is also safe and well-tolerated in children, with clinical effects barely affected by age.17,35 Single-allergen SLIT has also been confirmed to be effective in both monosensitized and polysensitized patients. SLIT may exhibit long-term efficacy and prevent new sensitization. It is generally considered that patients could acquire relatively stable therapeutic effects after three years of SLIT.26 Strengthening effective education about treatment and using motivational communication strategies, particularly in the initial stage of SLIT, can significantly increase patient compliance.
This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801) and the Health Promotion Project of Jiangsu Province (XK200719, RC2007065 and RC2011071), the People's Republic of China. We would like to thank Professor De Yun Wang at the Department of Otolaryngology, Yong Loo Lin School of Medicine, National University of Singapore for kind advice in the manuscript preparation.
Conflict of interestThe authors have no conflict of interest to declare.