Asthma is the most common chronic illness in childhood and really affects the everyday life of patients who suffer from it. Since asthma is a common disease, there is a great endeavour to achieve the most appropriate treatment option. Despite inhaled corticosteroids and leukotrien receptor antagonists both being routinely used in asthma treatment, specific immunotherapy is still questioned. There are numerous aspects affecting asthma-related quality of life, such as age; seasons; disease control and severity etc, which are well studied -apart from the type of treatment. With this study we aimed to stress the influence of asthma treatment on quality of life.
MethodsA total of 102 children, aged 6–18 years, were assigned to classic asthma therapy (n=50) and specific immunotherapy (n=52). The quality of life is assessed using the Standardized Pediatric Asthma Quality of Life Questionnaire (PAQLQ) interviewer-administered Turkish version. Pulmonary function testing was performed on the same day, after the questionnaire was completed.
ResultsThe PAQLQ total scores were significantly higher in the specific immunotherapy group (p<0.001). Apart from emotional function domain scores; symptoms domain and activity limitation domain scores were higher in the specific immunotherapy group. Emotional function domain scores were similar in the two groups (p>0.05). There were no statistically significant differences in pulmonary function testing results between the two groups (p>0.05). There was a linear correlation between FEV1%, FVC level and total and domain scores of PAQLQ with Spearman Correlation tests.
Allergen specific immunotherapy is a specific therapeutic approach in the treatment of allergic airway diseases and insect venom allergy.1 Although allergen specific immunotherapy (SIT) has been used in the management of allergic diseases since 19112, the role of SIT in the treatment of paediatric asthma is still controversial. There is wide variation in practice around the world. Among the anti-asthma treatments available, SIT is still the only one that may modify the natural course of allergic asthma, because it interferes with the underlying immunological mechanism.1,3–5
Therefore, children with allergic airway diseases represent the age group most likely to benefit SIT. In theory, an intervention in early life may modify the development of the immune response to allergens, decreasing chronic inflammation and decline in lung function.6,18
Clinical efficacy of SIT in allergic rhino conjunctivitis and asthma has been confirmed in controlled trials.26 A beneficial effect for asthma was documented in several studies, showing over 40% improvement in symptom and medication scores.7,8 The efficacy has recently been confirmed in a meta-analysis.9 SIT has an allergen-specific modifying role in Th2 cell responses either by immune deviation (increase in Th0/Th1) or T-cell anergy (decrease in Th2/Th0) or both.10–12
Inhaled corticosteroids (ICS) are the most widely used anti-inflammatory medicine providing long-term prevention of asthma symptoms by suppressing, controlling and reversing inflammation in the airways.10 ICS reduce the survival of T-cells13 and thereby influence cytokine production and reverse airway inflammation.
To compare the benefits of SIT with pharmacotherapy, we re-evaluated a cohort of children previously diagnosed as having moderate allergic asthma. A sum of 102 asthmatic children who were treated with SIT (n=52) and pharmacotherapy (n=50), were enrolled to the study.
This study was designated for expressing the advantage of SIT on the improvement of quality of life and its corticosteroid sparing effect.
Material and methodsPatientsPatients were recruited from the outpatient clinic of Ankara Diskapi Children’s Hospital, Pediatric Asthma and Allergy Department.
Inclusion criteria were: age 7–18 years, allergy to house dust mite (HDM) (D. pteronyssinus or D. Farinae) or grass pollen species shown by positive skin prick test (>3mm) (Stallergenes); a clinical diagnosis of asthma suggested by symptoms such as episodic breathlessness, wheezing, cough and chest tightness responding to long-term treatment with inhaled corticosteroids (ICS; budesonide equivalent to 400–800mcg) and or LTB4 antagonists or SIT; and ability to perform spirometry. Asthma was diagnosed according to GINA guidelines.25
Exclusion criteria were:- 1.
Concomitant sensitisation to perennial allergens such as cockroach, Alternaria or Cladosporium mould species, cat, dog (if animal at home), and to seasonal pollen allergens inducing allergic symptoms lasting longer than four months/yr. Sensitisations were based on a clear-cut clinical history, positive skin tests, and specific IgE (RAST class of one or more, that is >0.35IU/ml).
- 2.
Previous immunotherapy with HDM extracts within three years from the date of inclusion.
- 3.
Contraindications to SIT, according to international guidelines.9
The first part of this study was retrospective and consisted of a file study, in which SIT-treated and control patients were selected according to strict criteria. Subjects were divided in two groups: allergen specific immunotherapy (SIT) group, and the pharmacotherapy (PT) group.
Thereafter, all subjects were invited to participate to the study. Clinical examination, Juniper’s paediatric asthma quality of life questionnaire, and lung-function testing were carried out during the re-evaluation and the number of asthma attacks in the last year was recorded from patients’ diaries. A further purpose of the present study was to evaluate potential reduction in the use of inhaled corticosteroids while SIT is added to the treatment in patients with moderate and severe asthma due to HDM and pollen allergy.
Quality of life assessmentAll subjects completed Juniper’s Pediatric Asthma Quality of Life Questionnaire (PAQLQ) by themselves. The PAQLQ developed by Juniper and colleagues was used to assess the effects of asthma on asthma-related quality of life (AQOL).14 The PAQLQ is an asthma-specific quality of life questionnaire designed to measure the impact of asthma on children’s daily life. The PAQLQ contains 23 questions (items) in 3 categories (domains) regarding activity limitations (5 items), symptoms (10 items), and emotional function (8 items). All patients answered the questions using a 7-point scale to record their level of impairment in the previous 2 weeks, where 1=maximum impairment, and 7=no impairment. The lower the PAQLQ score, the greater the impairment of AQOL.
SpirometrySpirometry was carried out using a computerised spirometer (Vmax20c, SensorMedics Co., Yorba Linda, California, USA), calibrated daily according to the current guidelines of the American Thoracic Society.24 Before spirometry, the patients were asked to withhold short-acting beta2-agonists for at least 6h, long-acting beta2-agonists for 12h, and theophyllines and leuketriene receptor antagonists for 48h.
Skin prick testsThe skin prick tests were performed with a panel of 10 of the most common aeroallergens (Stallergenes, France) by the use of standard prick method. The following antigens were applied to the skin of the forearm: cockroach, house dust, mixed trees, mixed grasses, polyvalent moulds, Dermatophagoides pteronyssinus (Dp), Dermatophagoides farinae (Df). Histamine hydrochloride 10mg/ml and allergen diluents were used as positive and negative controls, respectively. Wheal diameters of at least 3mm greater than those of the negative controls (15min after administration) were considered positive. The arithmetic mean of the two widest diameters of the wheal was calculated for statistical purposes.
Allergen specific immunotherapyThe up-dosing was performed during an 8-week period with two to three injections at each visit followed by maintenance treatment for 3 years. In the maintenance phase, patients received the individual maximum tolerated dose up to a maximum dose of 100 000 SQ-U (corresponding to 9.8lg major allergen Der p1/ml.). The interval between injections was 6±2 weeks. The patients in the placebo group received histamine dihydrochloride injections according to the same dose increase and maintenance schedule (0.00001, 0.0001, 0.001 and 0.01mg/ml). The regimen was modified in case of reaction to any injection, extended interval between administrations, undercurrent infection or worsening of asthma. In case of systemic reactions the up-dosing schedule was changed to a one injection per week schedule.
Adverse events related to immunotherapy:Small local reaction (<5cm swelling); larger local reaction (>5cm swelling); mild systemic reaction (itchy eyes, nose, throat or skin, dizziness); severe systemic reaction (laryngeal oedema, hypotension and respiratory distress).
There were no life-threatening severe reactions during the trial and six patients withdrew because of adverse events (five undercurrent illnesses and one worsening of condition attributed to mild systemic reaction).
Statistical analysisData were collected with SPSS for Windows 11.5 package programme. The distribution pattern of continuous variables was evaluated with Shapiro Wilk test. Definitive variables were defined as±standard deviation (min-max) and nominal variables as case number and percentage. Statistical significance between two groups was determined using student’s t test for normally distributed continuous variables and Mann Whitney U test for abnormal distributions. The magnitudes of linear correlation between continuous variables were calculated with Spearmen rho coefficient, and as significance. Nominal variables were analysed with Pearson x2 test and values were considered as statistically significant for p<0.05.
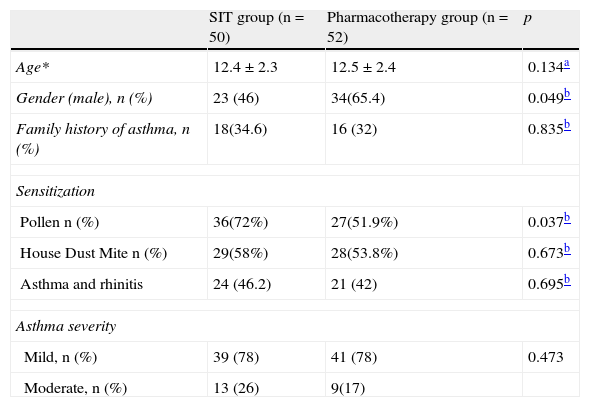
ResultsThe two groups were comparable with regard to baseline parameters, i.e. sex, age, dose of ICS, disease duration and PEF (Table 1). A total of 220 patients were willing to participate and were eligible for screening assessment, and 110 of them were randomly included into the study.
Baseline characteristics of thè study population
| SIT group (n=50) | Pharmacotherapy group (n=52) | p | |
| Age* | 12.4±2.3 | 12.5±2.4 | 0.134a |
| Gender (male), n (%) | 23 (46) | 34(65.4) | 0.049b |
| Family history of asthma, n (%) | 18(34.6) | 16 (32) | 0.835b |
| Sensitization | |||
| Pollen n (%) | 36(72%) | 27(51.9%) | 0.037b |
| House Dust Mite n (%) | 29(58%) | 28(53.8%) | 0.673b |
| Asthma and rhinitis | 24 (46.2) | 21 (42) | 0.695b |
| Asthma severity | |||
| Mild, n (%) | 39 (78) | 41 (78) | 0.473 |
| Moderate, n (%) | 13 (26) | 9(17) | |
*Datas are mean±SD.
One hundred and two of the 110 patients completed the study. Three patients in each group were withdrawn for the following reasons; six for personal reasons, two for developments of other diseases. Forty-five girls and 57 boys, in total 102 patients were recruited. Fifty-two of them were in the SIT group and the other 50 were in the PT group. The mean age was 12.4±2.3 (8–16) and 12.5±2.4 (8–18) for the SIT and PT groups, respectively. Patients in the SIT group were monosensitised either to pollen species (PS) or house dust mites (HDM). The PT group additionally had 13 polisensitised patients. The number of patients suffering from pollen sensitisation was statistically different in the PT group (n=36, p=0.037).
The mean number of asthma attacks emerged in a year was 5±3 (2–12) in the PT group and 1±1 (0–4) in the SIT group. There was a statistically significant difference in the number of acute asthma attacks between the two groups (p<0.001).
The mean duration of SIT was 33.9±11.5 (9–48) months. Forty-three (82.7%) patients were free of inhaled corticosteroids for a mean duration of 14.5±12.3 (3–48) months. Twenty-three patients (17.3%) from the SIT group still required pharmacotherapy in addition to SIT. No significant SIT related side effect was recorded.
PAQLQ score resultsGender had no impact on symptom, emotional, activity limitation domain and total scores of health quality. However, there was a linear correlation between age and symptom scores (p=0.015 and rho=0.239) but not between the other scores.
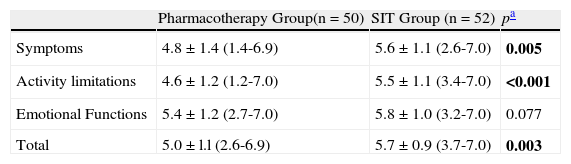
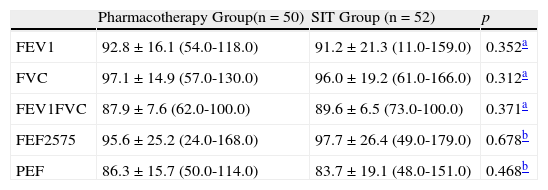
Symptoms and physical activity limitation domain and total health quality of life scores were statistically different between the two groups (p=0.05, p<0.001, p=0.003) (Table 3). Emotional scores were not significantly different (p=0.007). FEV1, FVC, FEV1/FVC, FEF25–75 and PEF values were similar between two groups (p=0.352, p=0.312, p=0.371, p=0.678 and p=0.468, respectively). (Table 2).
Between two groups in Pediatric Asthma Quality of Life Questionnaire scores
| Pharmacotherapy Group(n=50) | SIT Group (n=52) | pa | |
| Symptoms | 4.8±1.4 (1.4-6.9) | 5.6±1.1 (2.6-7.0) | 0.005 |
| Activity limitations | 4.6±1.2 (1.2-7.0) | 5.5±1.1 (3.4-7.0) | <0.001 |
| Emotional Functions | 5.4±1.2 (2.7-7.0) | 5.8±1.0 (3.2-7.0) | 0.077 |
| Total | 5.0±l.l (2.6-6.9) | 5.7±0.9 (3.7-7.0) | 0.003 |
Spirometry parameters between two groups
| Pharmacotherapy Group(n=50) | SIT Group (n=52) | p | |
| FEV1 | 92.8±16.1 (54.0-118.0) | 91.2±21.3 (11.0-159.0) | 0.352a |
| FVC | 97.1±14.9 (57.0-130.0) | 96.0±19.2 (61.0-166.0) | 0.312a |
| FEV1FVC | 87.9±7.6 (62.0-100.0) | 89.6±6.5 (73.0-100.0) | 0.371a |
| FEF2575 | 95.6±25.2 (24.0-168.0) | 97.7±26.4 (49.0-179.0) | 0.678b |
| PEF | 86.3±15.7 (50.0-114.0) | 83.7±19.1 (48.0-151.0) | 0.468b |
The primary analysis showed that treatment with SIT was numerically superior to the pharmacological group in its ability to cut off the use of ICS in allergic asthmatic children.
Allergen specific immunotherapy has been widely used to treat allergic asthma, although the introduction of effective inhaled therapies has changed the general pattern of asthma care. Current drug therapies for asthma suppress airway inflammation and relieve bronchospasm. None of these treatments are curative and asthma recurs rapidly on ceasing treatment.15–18 SIT is an immunomodulatoratory treatment mostly effective in selected asthma populations. Both treatments may alter T-cell dependent responses in asthma patients, SIT have an allergic-specific modifying role in Th2 cell responses either by immune deviation (increase in Th0/Th1) or T-cell anergy (decrease in Th2/Th0) or both. ICS reduce the survival of T-cells and thereby influence cytokine production and reverse airway inflammation.12,19
This study has demonstrated that SIT is an effective treatment for asthma in children and teenagers who are allergic to HDM or pollen species. We have shown that even after significant asthma is established in childhood, allergen specific immunotherapy has an important therapeutic role. HDM or pollen species allergy persists over many decades, causing systemic allergic symptoms and impairing quality of life in children. Because the clinical benefits of SIT have been shown to modify the long-term natural history of disease in adults, this treatment modality should be of particular interest in the paediatric population. In our study we discovered that SIT offers a disease and medication free future life to the majority of the patients. 82.7% of the SIT-treated patients were steroid-free in an average time of 14.5 months.
Therefore, these asthmatic children had been protected from the undesired side effects of corticosteroids. Blumberga et al. have studied the steroid sparing effect of SIT on adults and demonstrated that when SIT was introduced, the use of ICSs was significantly reduced, without loss of asthma control in patients with moderate persistent asthma.19 To our knowledge this is the first study in the literature which compares the impact of SIT and ICS on childhood asthma.
In the current era of health care, providers are increasingly taking into account HRQL outcomes as part of the decision-making process for the delivery of good quality care. As asthma is becoming increasingly prevalent, it is essential to study not only the causes and treatments of asthma, but also associated QOL. One of the purposes of this study is to examine whether the type of treatment has an influence on QOL (14, 20, and 21).
Quality of life used to be based on the conventional assessment of asthma severity, on pulmonary function testing, on the presence and intensity of symptoms or need for medication in the asthmatic children. Past studies have demonstrated a varied relationship among these parameters.14,20,21 The percentage of predicted FEV1 was correlated with asthma attacks and symptom score but not with number of days with symptoms. FEV1% was correlated with asthma related QOL in mild asthmatics, but not in severe asthmatics. In a paediatric population, asthma symptoms have been correlated with asthma related QOL but not FEV1%. Boran et al. also demonstrated that there is a statistically significant correlation between HQOL scores and pulmonary function testing.21 Similarly, we have demonstrated a linear correlation between FEV1% and HQOL scores.
Pifferi et al. studied the benefits of immunotherapy in asthmatic children in a three-year prospective trial and showed that the number of asthma exacerbations significantly decreased among SIT patients compared to controls.22 We also statistically confirmed this data and suggest that SIT is a successful treatment in order to decrease asthmatic symptoms and control the disease activity.
Asthma patients have an increased risk of developing acute systemic side effects after SIT.23 No life-threatening reactions occurred during the study and no subject was withdrawn from allergen specific immunotherapy as a result of adverse effects or perceived lack of efficacy. In general, the low incidence of side effects in this study may be explained by careful dosing, i.e. evaluation of the patients after each injection and meticulous attention to health status before each injection.
The aim of this present study was to assess the effect of SIT -whether it avoids children from corticosteroids or their side effects and advances health quality of life. This study suggests that allergen specific immunotherapy might have a steroid-sparing anti-inflammatory effect.
Conflict of interestThe author no conflicts of interest.