Allergic rhinitis is the most common allergic disease with negative impacts on patients’ quality of life. The prevalence and pattern of sensitisation vary between different countries and populations. Identifi cation of the most prevalent aeroallergens in each area has a very important role in diagnosis and treatment of allergic rhinitis.
Iran is a fairly large country with different geo-climatic conditions and there are no data about the most prevalent aeroallergen in Mashhad City, which is the second largest city in Iran, with a semiarid climate. The aim of this study was to evaluate the prevalence of positive skin test to various common aeroallergens among allergic rhinitis patients in the city of Mashhad.
Materials and MethodsSkin prick tests were performed with 27 common regional aeroallergens including grass, weed, tree, mite and mould in 311 patients with allergic rhinitis.
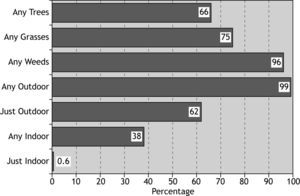
ResultsThe overall rate of sensitisation to any allergen was 81 %. 76 % of patients were poly-sensitised and weed and grass were the most prevalent allergens (77 % and 62 % respectively). Salsola Kali and mould were the most and the least prevalent individual allergens (72.5 % and 6.5 % respectively). Mean total IgE in patients with positive skin prick test was signifi cantly higher than in patients without any positive skin prick test (308 vs 128IU/mL, P=0.016). 97 % of atopic patients were sensitised to Salsola kali and or Fraxinus americana.
ConclusionsOur work showed the importance of weeds, especially the Amaranthaceae and Chenopodiaceae families. Diagnosis of pollen allergy can be simplifi ed by using a combination of a few common aeroallergens.
Allergic rhinitis is the most common allergic disease and many epidemiological studies have revealed a progressive increase in the prevalence during recent decades 1,2. The prevalence of allergic rhinitis varies considerably across countries and regions and, according to the International Study of Asthma and Allergies in Childhood 1, it ranges between 1.4 % and 45 % of the total country population3.
Although it is not usually a severe disease, direct and indirect effects of allergic rhinitis including cost of treatment, impaired quality of life and presence of co-morbidities cause significant impact on the public health system4,5.
Allergic rhinitis is characterized by nasal symptoms including sneezing, runny nose, itching and nasal congestion. Aeroallergens, especially pollen, are the most important factors which cause symptoms in allergic rhinitis6.
Avoiding allergen exposure is the first step in the manage-ment of allergic rhinitis although it is not straightforward. However, identification of the full spectrum as well as the most common aeroallergen to which the patient responds in each area still has a very important role in diagnosis and treatment of allergic rhinitis. Choosing the most reliable and the most cost effective panel of allergen extracts for skin prick test (SPT) as the most appropriate diagnostic test, and finding the best formulation of inhalant allergen immunotherapy as an effective treatment, strictly depend on information about the most important aeroallergens in each area.
Many studies have shown that the distribution and pattern of aeroallergens is significantly different in different countries7 and even in different parts of a country8.
Iran is a fairly large country with different geo-climatic conditions in different regions. Mashhad is the second largest city in Iran, located in the Northeast with a semiarid climate, hot summers and cold winters (mean annual temperature = 16°C, range –32 to 41°C, average humidity = 50 and annual rainfall of 210mm). This climatic condition results in a specific pattern of vegetation which possibly differs from East Asia, most parts of Europe and the USA and subsequently it is possible that the pattern of sensitisation may be different in our atopic patients.
In a recent study we showed that the prevalence of allergic rhinitis is as high as 20 % in our area (manuscript in press) but in spite of this high prevalence, there are no data regarding the most important aeroallergens in the same area.
The purpose of this study was to evaluate the prevalence of positive skin test to various common aeroallergens among allergic rhinitis patients in the city of Mashhad, in order to develop better strategies for prevention, management and treatment of allergic rhinitis.
Materials and MethodsStudy population and designThis study was conducted in the Immunology Research Centre of Mashhad University of Medical Sciences, Mashhad, Iran. The study group was constituted from 356 patients with rhinitis symptoms, who were referred to our research centre between April to October 2006 from outpatient clinics in random. The study was approved by the Ethics Committee of the Immunology Research Centre, Mashhad, Iran and all patients provided informed consent to participate in the study.
In total 311 patients were included in the study if they met inclusion criteria and agreed to participate. Inclusion criteria were: (1) diagnosis of current allergic rhinitis as defined by having a problem with sneezing or a runny or blocked nose in the absence of a cold or flu in the past 12months; (2) non-treated at the time of inclusion, and; (3) negative history of specific immunotherapy.
Data about demographic variables, smoking history and family history of allergic diseases were obtained by a questionnaire. Allergic family history was positive when the patient had one or more relatives who had suffered from any allergic disease. Active smokers where defined as those who have smoked at least one cigarette per day or one cigar per week for a period of one year and continue to do so. Current rhino-conjunctivitis was defined as the simultaneous presence of allergic eye symptoms with current allergic rhinitis.
Skin prick testSkin prick tests with 27 common regional allergen extracts (HollisterStier, USA) were performed in all patients by a physician, according to European guidelines9. Allergens used in this study were chosen according to common regional plant species and included 6 grasses (Cynodon dactylon, Poa pratensis, Sorghum halepense, Dactylis glomerata, Lolium perenne and Phelum pratense), 8 weeds (Amaranthus palmeri, Xanthium strumarium, Kochia scoparia, Amaranthus retroflexus, Salsola kali, Artemisia vulgaris, Rumex acetosella and Chenopodium album), 8 trees (Acacia longifolia, Fraxinus americana, Juniperus ashei, Populus deltoides, Cupressus arizonica, Pinus spp, Platanus occidentalis and Ailanthus altissima), 3 moulds (Alternaria tenuis, Aspergillus fumigatus and mould mix), 2 mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae) and one cockroach mix extract (Periplaneta americana and Blattela germanica). Histamine hydrochloride (10mg/mL) and glycerol saline were used as positive and negative controls, respectively. The mean wheal size was recorded after 15 minutes and SPT was regarded as positive with a wheal size of minimum 3-mm larger than the negative control. Three patients were excluded from the study because of the positive response to negative control and two were excluded because they were pregnant.
Total IgEVenous blood was collected from 257 (84 %) patients and centrifuged at 2500rpm for 10 minutes. Serum samples were stored at —20°C until tested. Total IgE was determined in serum samples in duplicate with a commercially available enzyme immunoassay kit (Radim, Italy) according to the manufacturer's instructions. Based on the kit's manual, all values higher than 100IU/mL were considered as high total IgE.
Statistical analysisData were analyzed by SPSS software package version 11 (Chicago, USA). Chi-squared test was used for comparison of frequencies. Total IgE is presented as geometric mean with 95 % confidence interval. P value less than 0.05 in the two-tailed test was considered significant.
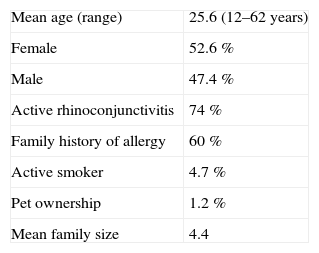
ResultsIn this study, 306 allergic rhinitis patients were evaluated for their skin prick test response to 27 common regional aeroallergens. Demographic characteristics of patients are shown in Table I. Among the patients, 226 (74 %) had current allergic rhinoconjunctivitis, and a positive family history of allergy was found in 184 cases (60 %).
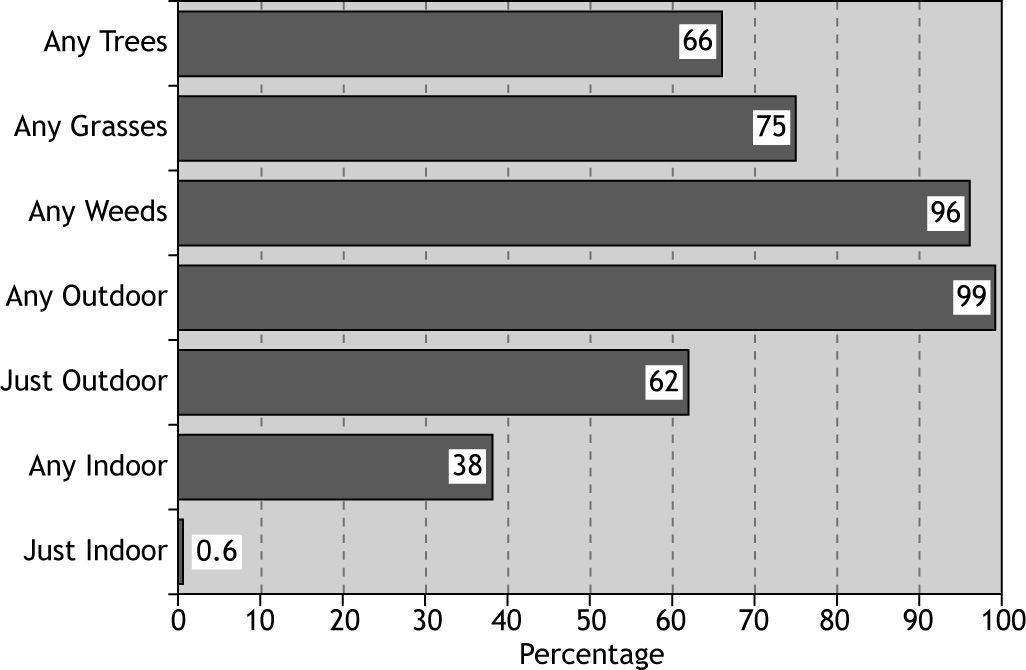
The overall rate of sensitisation to any allergen was 81 %, and 19 % of patients did not show positive skin response to any of tested allergens (Fig. 1). The most prevalent aeroallergen category was weeds (77 %) followed by grasses and trees, and the least prevalent was moulds (11.3 %) (Fig. 1).
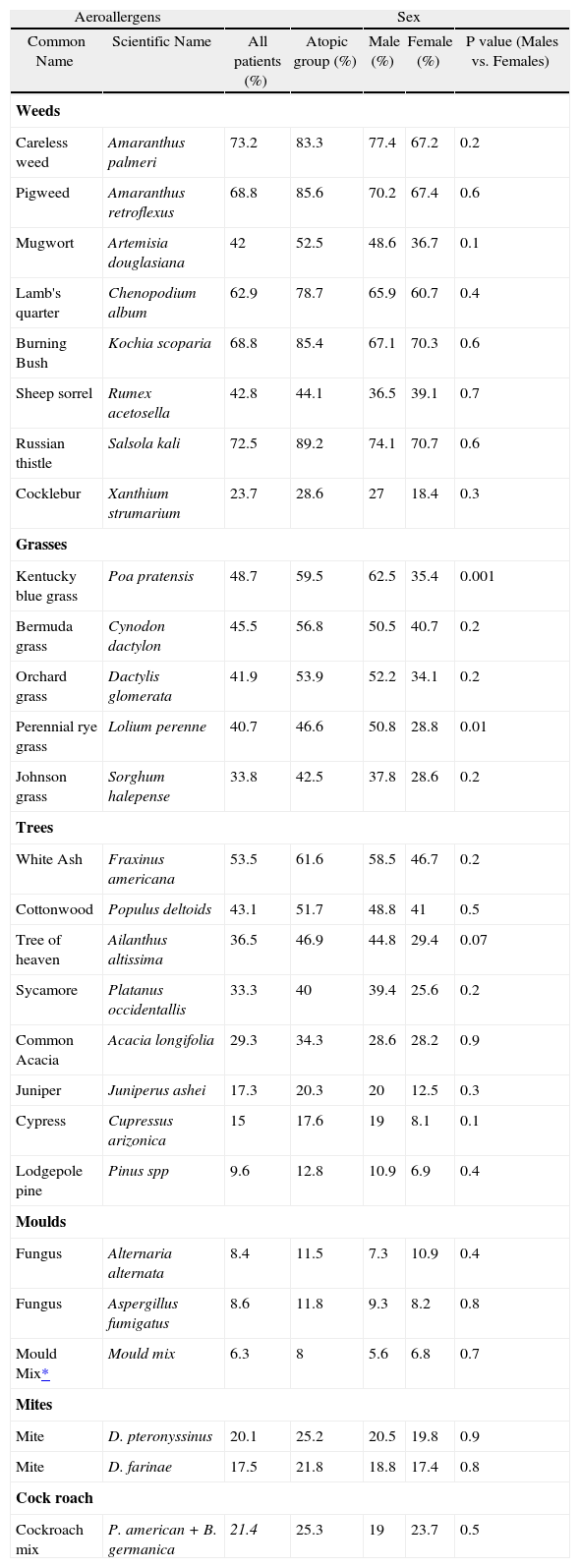
Amaranthus palmeri, Salsola kali, Kochia scoporia, Amarantus retroflexus and Chenopodium album were the most prevalent allergens among allergic rhinitis patients (73.2 %, 72. 5, 68.8 %, 68.8 % and 62.9 %, respectively).
Among tree pollen, the most prevalent allergen was Fraxinus americana (53.5 %) and the least prevalent was Pine spp (9.6 %).
The most and the least frequent indoor allergens were cockroach and mould mix (21.4 % and 6.3 %, respectively) (Table II).
Prevalence of positive skin prick test and total IgE among allergic rhinitis patients
| Aeroallergens | Sex | |||||
| Common Name | Scientific Name | All patients (%) | Atopic group (%) | Male (%) | Female (%) | P value (Males vs. Females) |
| Weeds | ||||||
| Careless weed | Amaranthus palmeri | 73.2 | 83.3 | 77.4 | 67.2 | 0.2 |
| Pigweed | Amaranthus retroflexus | 68.8 | 85.6 | 70.2 | 67.4 | 0.6 |
| Mugwort | Artemisia douglasiana | 42 | 52.5 | 48.6 | 36.7 | 0.1 |
| Lamb's quarter | Chenopodium album | 62.9 | 78.7 | 65.9 | 60.7 | 0.4 |
| Burning Bush | Kochia scoparia | 68.8 | 85.4 | 67.1 | 70.3 | 0.6 |
| Sheep sorrel | Rumex acetosella | 42.8 | 44.1 | 36.5 | 39.1 | 0.7 |
| Russian thistle | Salsola kali | 72.5 | 89.2 | 74.1 | 70.7 | 0.6 |
| Cocklebur | Xanthium strumarium | 23.7 | 28.6 | 27 | 18.4 | 0.3 |
| Grasses | ||||||
| Kentucky blue grass | Poa pratensis | 48.7 | 59.5 | 62.5 | 35.4 | 0.001 |
| Bermuda grass | Cynodon dactylon | 45.5 | 56.8 | 50.5 | 40.7 | 0.2 |
| Orchard grass | Dactylis glomerata | 41.9 | 53.9 | 52.2 | 34.1 | 0.2 |
| Perennial rye grass | Lolium perenne | 40.7 | 46.6 | 50.8 | 28.8 | 0.01 |
| Johnson grass | Sorghum halepense | 33.8 | 42.5 | 37.8 | 28.6 | 0.2 |
| Trees | ||||||
| White Ash | Fraxinus americana | 53.5 | 61.6 | 58.5 | 46.7 | 0.2 |
| Cottonwood | Populus deltoids | 43.1 | 51.7 | 48.8 | 41 | 0.5 |
| Tree of heaven | Ailanthus altissima | 36.5 | 46.9 | 44.8 | 29.4 | 0.07 |
| Sycamore | Platanus occidentallis | 33.3 | 40 | 39.4 | 25.6 | 0.2 |
| Common Acacia | Acacia longifolia | 29.3 | 34.3 | 28.6 | 28.2 | 0.9 |
| Juniper | Juniperus ashei | 17.3 | 20.3 | 20 | 12.5 | 0.3 |
| Cypress | Cupressus arizonica | 15 | 17.6 | 19 | 8.1 | 0.1 |
| Lodgepole pine | Pinus spp | 9.6 | 12.8 | 10.9 | 6.9 | 0.4 |
| Moulds | ||||||
| Fungus | Alternaria alternata | 8.4 | 11.5 | 7.3 | 10.9 | 0.4 |
| Fungus | Aspergillus fumigatus | 8.6 | 11.8 | 9.3 | 8.2 | 0.8 |
| Mould Mix* | Mould mix | 6.3 | 8 | 5.6 | 6.8 | 0.7 |
| Mites | ||||||
| Mite | D. pteronyssinus | 20.1 | 25.2 | 20.5 | 19.8 | 0.9 |
| Mite | D. farinae | 17.5 | 21.8 | 18.8 | 17.4 | 0.8 |
| Cock roach | ||||||
| Cockroach mix | P. american + B. germanica | 21.4 | 25.3 | 19 | 23.7 | 0.5 |
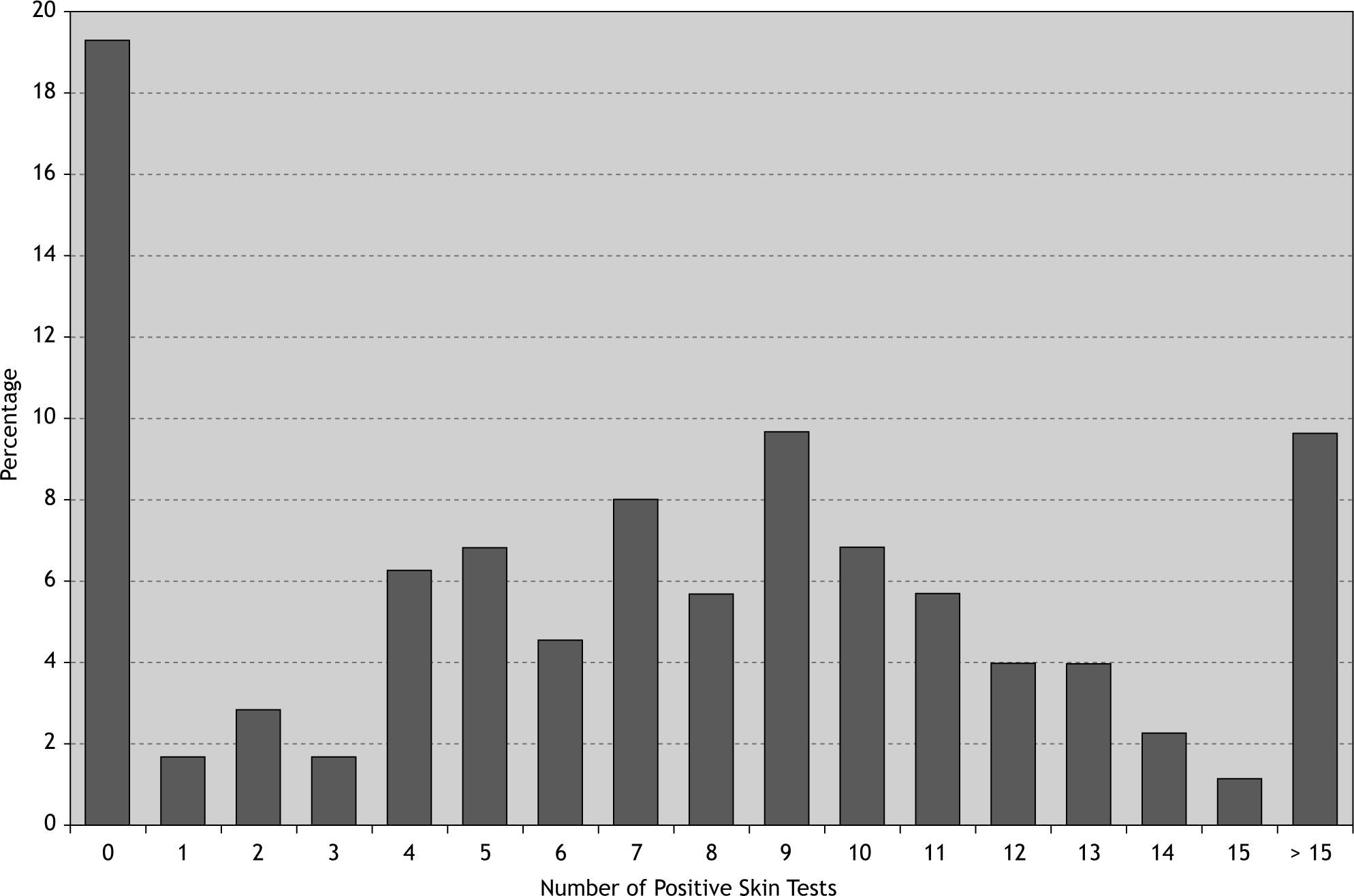
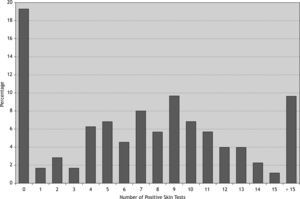
76.1 % of patients were poly-sensitised (positive skin reaction to more than three allergens) and about 33 % of them were sensitised to more than ten different allergens (Fig. 2). Among the patients with at least one positive SPT the most and the least prevalent allergens were Salsola kali and mould mix (89 % and 8 %, respectively). 40 % of this group were sensitised to more than 10 different allergens (Table II). Interestingly 97 % of atopic patients had positive SPT to Salsola kali and/or Fraxinus americana.
There was a significant difference between male and female patients in the rate of sensitisation to Poa pratensi, Dactylis glomerata and Lolium perenne (P < 0.05).
The mean total IgE serum was 278IU/ml. Males had higher mean total IgE values than females (305 vs 252IU/mL, P = 0.6), but the difference was not significant. Patients with positive SPT had significantly higher values than patients without positive SPT (308 vs 128IU/mL, P = 0.016).
DiscussionAeroallergens are the most important triggers of allergic symptoms in allergic rhinitis. Finding the most prevalent allergen has been the subject of many studies throughout the world, but studies in different parts of the world could not show a unique pattern of sensitisation. Common aeroallergens and particularly pollen in each area significantly depend on geo-climatic conditions10 and as such, allergens used for SPT or specific allergen immunotherapy should be selected on the basis of prevalence in the patient's geographic area.
In this study we showed the importance of pollen and in particular weed pollen as a trigger of allergic symptoms in our area. In some countries, indoor allergens are very important11,12 but generally pollens are the most common causes of allergic rhinitis13 while the most important pollens are different in each area.
All the five most common aeroallergens in our study were weeds, from Amaranthaceae and Chenopodiaceae families which are botanically close and a have high degree of cross-reactivity, possibly because of the presence of common allergenic determinants14.
These weeds are found throughout the world, especially in North America and some parts of Europe. They are highly adaptable and drought-tolerant and found on saline soils, deserts and coasts. Our results are in agreement with other studies from the USA15, Thailand16 and Spain17 which showed the importance of weed pollen. However, we have higher rates of sensitisation in comparison to western countries, being more similar to result of surveys from Saudi Arabia18, Kuwait 19 and United Arab Emirates 20 in the Persian Gulf area.
The rate of sensitisation to grass pollen in this study was 45 % which is less than some other studies, mostly from European countries such as the Netherlands21 and Germany22, but higher than the results of some studies from our neighbouring countries like Saudi Arabia18 and United Arab Emirates 20 which have found grass pollen as very prominent sensitising allergens.
In our study some tree pollens like Fraxinus sp. and Populus sp. were fairly prevalent similar to the studies of Spain23 Saudi Arabia18 and Turkey24 and in contrast to studies from Hungary25 and the USA26 which showed a lower rate of sensitisation to tree pollen. Pollens of Cupresssaceae and Pinaceae families were not common, in contrast to the reports from the USA 27 and Italy 28, and similar to a study from Spain29.
The sensitisation rate to tree pollen is highly variable depending on the tree population and level of exposure. Some studies report cross-reactivity between different tree pollens and also grass/weed pollen30,31 suggesting the presence of some common epitopes in unrelated plants and it is possible that it is atopic patients' response to some shared epitopes.
In the current study, the highest rate of sensitisation to house dust mites and moulds was found to be 20 % and 9.2 %, respectively, which was lower than the results of other studies from humid area such as Malaysia32 and Thailand16 and along the lines of two other studies conducted in Iran 33,34. With consideration of optimum condition for mite and mould growth, our result were to be expected and in a recent study about house dust mite allergens in seven different cities throughout Iran (unpublished data) supported our results because we could not detect any mite allergens in dust samples from Mashhad homes. The explanation for low rate of sensitisation despite the absence of apparent mite infestation could be previous exposure in another place or a cross-reaction with cockroach because of the presence of tropomyosin as a major allergen in both species35.
Surprisingly, mite allergens were also reported in hot and dry regions, like Kuwait19 and Sistan and Baluchestan province of Iran36. It might be related to wide use of air conditioners which make good conditions for mites to grow and increase susceptibility to indoor allergens.
In our study over 90 % of atopic allergic rhinitis patients were poly-sensitised to more than three allergens. In a study in Poland, hypersensitivity to grass, tree and/or shrub pollen coexisted in 85 % of patients37 and a similar study in Kuwait showed that up to 65 % of all sensitised subjects were positive for more than one allergen19. Poly-sensitisation toward several allergens might be because of various factors such as genetic38 and environmental factors, which favour growth and vegetation of specific plant species like grass and weeds with similar survival conditions. It might also reflect the presence of common allergenic epitopes in different but botanically close plant species, or the presence of panallergens in distantly related plants. We used a number of closely related species which may create a high rate of poly-sensitisation but more than a third of patients were sensitised to more than 10 allergens, which shows co-sensitisation between different categories of allergens.
The mean total IgE was high among allergic rhinitis patients, as other studies have shown high level of total IgE in allergic patients39 although the levels of total IgE depend on many other factors; such as parasitic infestations, smoking, pollution, local diet and different genetic background40.
In spite of our strict inclusive criteria in this study, about 19 % of patients did not show any skin responses. This is lower than some other studies which reported that 30 %41 and 58 %42 of hay fever patients were seronegative. Because of the sensitivity of SPT which is less than 100 %, some symptomatic patients can be negative in SPT and some authors claim that mild to moderate levels of hypersensitivity may not be detected by SPT alone 43. It is also possible that the patients were sensitised to particular allergens which have not been tested in our study; or not identified yet; or some non-allergic forms of rhinitis included among our cases.
Our results about the importance of pollen, especially weed and grass pollen can be explained by consideration of the geo-climatic parameters in our area. Studies have shown that temperature, precipitation, relative humidity and atmospheric CO2 influence pollen production and concentration in the atmosphere and consequently increase exposure to pollen, although there is a body of evidence suggesting racial differences in the prevalence of sensitisation to specific allergens44,45. Because of the lack of aerobiological information about the atmospheric load of different pollen in our area, we cannot conclude whether the high prevalence of grass/weed sensitisation is related to the high exposure rate or the high potency of allergens in grass/weed pollen.
This study evaluated a large panel of aeroallergens in a large number of highly selected allergic rhinitis patients in their active phase of the disease but cat and dog allergens were not included as routine items in our SPT since, as we have shown in another study, keeping pets in the home is not common in Iran.
In conclusion, our work showed the importance of weed/ grass especially Amaranthaceae and Chenopodiaceae families. The results suggest the use of these allergens in any diagnostic or treatment strategy for the management of allergic rhinitis patients. Diagnostics of pollen allergy can be simplified by using a combination of a few common aeroallergens (e.g., Salsola spp and Fraxinus spp).
FundingThis study was made possible by a grant from the Research vice presidency of Mashhad University of Medical Sciences, Mashhad, Iran.
Conflict of interestThe authors have no conflict of interest to declare.
This study was made possible by a grant from the Research vice presidency of Mashhad University of Medical Sciences. The authors wish to thank Dr Adriano Mari (Center for Clinical and Experimental Allergology, Rome, Italy) and Dr Simon Royce (Murdoch Children's Research Institute, Melbourne, Australia) for critical review of the manuscript and the study participants for their cooperation.