Yellow dye tartrazine is a potential cause of exacerbations of asthma, allergic rhinitis and urticaria in atopic patients. The Brazilian Sanitary Surveillance Agency (ANVISA) published a consultation about the possibility of issuing a label warning addressing these potential effects of food and drugs containing tartrazine. The present study aims to evaluate tartrazine dye safety in atopic subjects suffering from allergic rhinitis, asthma, urticaria or sensitivity to non-steroidal anti-inflammatory drugs (NSAIDs).
MethodsAtopic patients with allergic rhinitis, asthma, urticaria or pseudo-allergic reactions to non-steroidal anti-inflammatory drugs were studied (n=26). The gold standard, double-blind placebo controlled, crossed-over challenge was used
ResultsThere were no statistical differences between placebo and drug in cutaneous, respiratory or cardiovascular aspects.
ConclusionsIn a group of atopic subjects with allergic rhinitis, asthma, urticaria or pseudo-allergic reactions to non-steroidal anti-inflammatory drugs, the administration of 35mg of the tartrazine dye did not precipitate any kind of significant cutaneous, respiratory or cardiovascular reactions when compared to placebo.
Chemical compounds, generically named additives, are frequently present as components of nourishing products and medicines. One of these widely used additives, yellow tartrazine, is a matter of controversy regarding its potential to cause adverse effects in subjects who suffer from allergic rhinitis, asthma, urticaria and sensitivity to non-steroidal anti-inflammatory drugs. ANVISA published a consultation about the possibility of issuing a label warning addressing these potential effects of food and drugs containing tartrazine. Lockey1 was the first author who reported a possible association between tartrazine and urticaria in three urticaria patients. Other subsequent communications had associated exacerbations of asthma, urticaria and anaphylaxis to the additive use in foods and medicines2–4.
Ram and Ardern5 accomplished a systematic review of the published studies about the role of tartrazine on asthma exacerbations. Ninety studies which analysed groups of asthmatic patients who had been subject to controlled exposition to tartrazine were found. Eighty-four of them were excluded from the review due to methodological problems. From the six remaining studies, authors concluded that there is no irrefutable indication that abstinence from the use of tartrazine dye could benefit asthma patients.
The present study aims to evaluate tartrazine dye safety in atopic subjects suffering from allergic rhinitis, asthma, urticaria or sensitivity to non-steroidal anti-inflammatory drugs (NSAIDs).
Patients and methodsPatients were recruited from an adult population (18–65 years old) attending specialised services in Allergy, Asthma and Immunology in Antonio Pedro University Hospital, and from people answering to a posted invitation. The protocol has been performed in accordance with the Declaration of Helsinki. The Ethical Committee of the Medical School approved it, and all subjects gave written, informed consent. The initial inclusion criteria were medical diagnosis of at least one of the following conditions: allergic rhinitis (intermittent/persistent) or ≥2 attacks of asthma or urticaria in the last two years. Those subjects with a history of sensitivity to acetyl salicylic acid or NSAIDs with two or more attacks of respiratory, cutaneous or cardiovascular symptoms were also included. All the included individuals were atopic, that is, showed two or more positive prick tests in a battery of seven aeroallergens (IPI-ASAC, Brazil) including mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae and Blomia tropicalis), moulds mixture (Aspergillus fumigatus+Penicillium notatum+Alternaria alternata+Cladosporium herbarum), grass mixture (Dactylis glomerata+Festuca pratensis+Lolium perenne, Phleum pretense, Poa pratensis) and dander of cat and dog. Wheals of 3mm or greater, as compared to saline, were considered positive.
Subjects with a history of anaphylaxis, hereditary forms of angio-oedema, asthma dependent on systemic steroids, other pulmonary conditions and those using beta blockers drugs or suffering from dermatologic diseases that could interfere with cutaneous evaluation were excluded. Those with non-allergic rhinitis or chronic sinusitis were also not accepted. Fasting glucose tests ≥100mg/dl and serum creatinine ≥1.5mg/dl also served as exclusion criteria. At the time of testing, patients had no significant active medical conditions.
Of the twenty-six patients who completed the study, 24 (92.3%) suffered from rhinitis (nine had mild intermittent, four mild persistent, five moderate intermittent, and six moderate persistent) associated or not with the other studied conditions. Asthma was present in 11 patients (42.3%) being mild intermittent in six, mild persistent in one, and moderate persistent in four. Six subjects (23.0%) suffered from urticaria (three of them of the mild chronic type and three with the intermittent acute type). Five patients (19.2%) had NSAIDs sensitivity. Fourteen patients (53.8%) presented two or more of the studied allergic diseases. Six subjects were on regular intranasal steroids and three on orally inhaled steroids; two of these cases were also using long acting bronchodilator. Subjects were allowed to maintain their regular medication. With respect to prick tests, all patients except one, had positive test to Dermatophagoides pteronyssinus and only one patient had positive test to grass mixture. The tests were positive to Dermatophagoides farinae in 20 patients (77%); to Blomia tropicalis in 20 (77%); to moulds mixture in 5 (19%); to dander of cat in 6 (23%) and to dander of dog in 5 (19%). None of the patients had past history of clinically documented reactions of any type to yellow food or drugs.
The protocol adopted the double-blind placebo controlled cross-over challenge (DBPCC), considered a gold standard method in the diagnosis of allergic reactions to food and drugs6,7. Capsules were manufactured by an external pharmacist who maintained the code until all the challenges were completed. Briefly, each volunteer was challenged either with tartrazine (Yellow dye no. 5, FD & C) in one visit, or placebo (talc) on another visit, one week apart. In the first visit, patients were randomised to receive three identical opaque capsules containing tartrazine or placebo (talc) in three steps. The administered dose of tartrazine was progressively increased from 5mg in the first administration to 10mg in the second one and to 20mg in the last one.
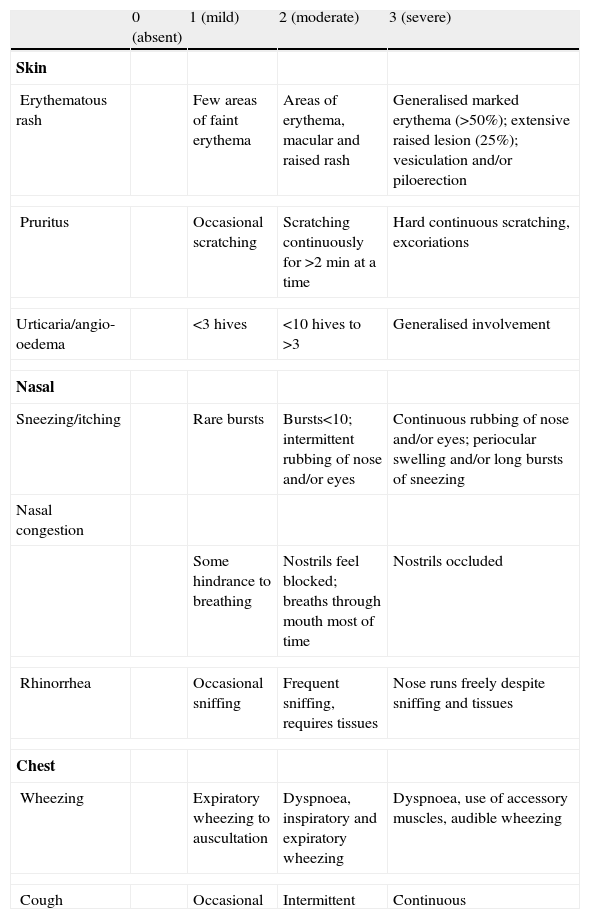
In the second visit, subjects who had taken the study drug received placebo and vice-versa. Randomisation, in blocks of ten, was made by raffle. Doses were administered within 60min intervals and were preceded by clinical examination. Symptoms were scored by evaluating skin, nasal and chest manifestations as suggested by Bock6 (Table 1). The score ranges from 0 to 9 (cutaneous and nasal) and 0 to 6 (thoracic) with numbers increasing as a reflex of the severity of clinical findings. A sequential Peak Expiratory Flow Rate (PEFR) assessment was performed and the best of three measures was registered at each clinical evaluation. All the eligible subjects were trained in the use of a Peak-Flow meter monitor (Mini Wright, Clement Clark, U.K.).
Symptoms score sheeta
| 0 (absent) | 1 (mild) | 2 (moderate) | 3 (severe) | |
| Skin | ||||
| Erythematous rash | Few areas of faint erythema | Areas of erythema, macular and raised rash | Generalised marked erythema (>50%); extensive raised lesion (25%); vesiculation and/or piloerection | |
| Pruritus | Occasional scratching | Scratching continuously for >2min at a time | Hard continuous scratching, excoriations | |
| Urticaria/angio-oedema | <3 hives | <10 hives to >3 | Generalised involvement | |
| Nasal | ||||
| Sneezing/itching | Rare bursts | Bursts<10; intermittent rubbing of nose and/or eyes | Continuous rubbing of nose and/or eyes; periocular swelling and/or long bursts of sneezing | |
| Nasal congestion | ||||
| Some hindrance to breathing | Nostrils feel blocked; breaths through mouth most of time | Nostrils occluded | ||
| Rhinorrhea | Occasional sniffing | Frequent sniffing, requires tissues | Nose runs freely despite sniffing and tissues | |
| Chest | ||||
| Wheezing | Expiratory wheezing to auscultation | Dyspnoea, inspiratory and expiratory wheezing | Dyspnoea, use of accessory muscles, audible wheezing | |
| Cough | Occasional | Intermittent | Continuous | |
Patients were admitted at 7:30 a.m. All had fasted for 12h and were interviewed about any clinical complaints and rescue drugs used in the previous week. Subsequently, they were submitted to a baseline evaluation which consisted of a clinical examination for scoring of skin, nasal and chest manifestations, and PEFR measurement. Patients received a dye–free, nutritionally balanced meal at 8:00 a.m. At 9:00 a.m., the first capsule (placebo or drug) was ingested with half a glass of fresh water. Two other dye-free meals were offered during the study at 10:30 am and 1:00 pm. Fifty minutes after the ingestion of each capsule, and 2h after the last one, the subject was clinically examined. Vital signs were also clinically monitored. The procedure was planned to be interrupted in case of appearance of severe symptoms. All patients stayed under observation for three hours after taking the last dose. All the subjects answered a questionnaire about symptoms after this period and were interviewed by phone the next day.
Statistical analysisThe sample size was estimated by the differences between scores obtained at baseline and in the 4th evaluation. Setting the statistical power at 0.8 and alpha at 0.05, we defined a total of 24 subjects as the minimal number of patients. Symptom scores were expressed as the mean of the total sum of individual score (TSS) for cutaneous, nasal and thoracic manifestations. Differences between the mean TSS at baseline and the last evaluation for placebo and tartrazine as well as differences between treatments were evaluated by use of Wilcoxon signed rank test. p values below 0.05 were considered statistically significant.
ResultsOne hundred and ten patients answered the invitation to participate. Sixty showed up to the first interview. Thirty-four were excluded after application of the exclusion criteria or by not showing up for the initial challenge. Twenty-six completed the DBPCC (17 females/9 males). The medium age was 37 years (18–63). None of the patients had been in regular use of antihistaminic drugs for the previous 7 days.
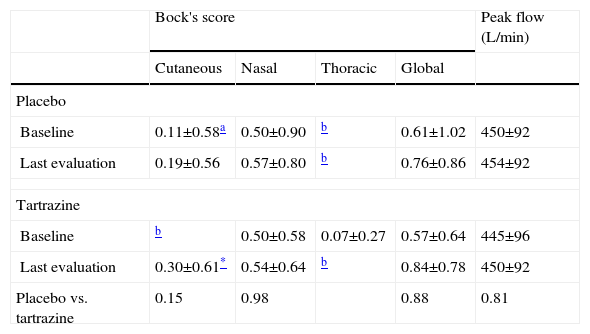
As a whole, responses to the challenges did not elicit clinical reactions requiring drug treatment (Table 2). Indeed, it should be noticed that all mean score values were very low seating below 1, denoting that clinical findings did not reach what is referred to by Bock3 as a mild level.
Challenge results from placebo and tartrazine
| Bock's score | Peak flow (L/min) | ||||
| Cutaneous | Nasal | Thoracic | Global | ||
| Placebo | |||||
| Baseline | 0.11±0.58a | 0.50±0.90 | b | 0.61±1.02 | 450±92 |
| Last evaluation | 0.19±0.56 | 0.57±0.80 | b | 0.76±0.86 | 454±92 |
| Tartrazine | |||||
| Baseline | b | 0.50±0.58 | 0.07±0.27 | 0.57±0.64 | 445±96 |
| Last evaluation | 0.30±0.61* | 0.54±0.64 | b | 0.84±0.78 | 450±92 |
| Placebo vs. tartrazine | 0.15 | 0.98 | 0.88 | 0.81 | |
One patient presented with urticaria at baseline in the second visit, beginning at the same day. The symptoms were moderate, as we observed only five lesions on the legs, less than 2cm and with occasional pruritus. No rescue drugs were used. As the challenge progressed, the lesions remained restricted to the legs and pruritus disappeared.
When analysing clinical findings for each component of the adopted score, differences between mean baseline and mean last evaluation only reached statistical significance for the cutaneous score in the tartrazine challenge (p=0.02). Also, the mean post challenge score observed was only 0.30, far below the mild level of 1. For any individual component and for the global score, no statistical significance was found for baseline and post challenge values either in placebo or tartrazine treatment. Similarly, the comparisons between treatments, either at baseline or post challenge, did not reach statistical significance.
Mean values for PEFR did not change after challenge with placebo or tartrazine. Differences between treatments, either at baseline or post-challenge were also not significant.
DiscussionSince the description of the possible role of dyes in eliciting clinical reactions in atopic patients, concerns have been raised about their use in susceptible subjects. The controversial observations in the literature about the importance of the yellow dye found in drugs and food on allergic people led to a public consultation by ANVISA8 about the necessity of a warning on labels of tartrazine containing food and drugs. This impelled us to address the role of yellow dye in eliciting reactions in subjects suffering from atopic diseases. As the majority of the literature observations were uncontrolled, our study tried to address this issue by employing strict, validated methodology. The method of DBPCC was used as it is considered the gold standard to study food allergy. The design was double-blind and crossed over with the subjects being tested against the drug and the placebo in two occasions, one week apart.
A group of atopic subjects suffering from asthma, allergic rhinitis, and urticaria or with sensitivity to NSAIDs and showing at least two positive skin prick tests to a battery of common inhalant allergens was selected for the study.
The doses of tartrazine were similar to those used by other authors in recent studies9,10. We have based the dose on the maximal quantity of tartrazine permitted by ANVISA in 100ml of soft drink and 100mg of snacks, which should be 0.01g/100ml and 0.02g/100g, respectively. The estimated mean daily intake of tartrazine in French adults was 0.4mg/kg bw/day11. The total dose of 35mg was used in our study.
One could question if the evaluated parameters have accuracy for the study of the various aspects of the clinical challenge. We shall see that all data in the literature point to cutaneous, respiratory or cardiovascular symptoms when a clinical reaction is observed6,9,12. Regarding dermatologic symptoms; pruritus, erythema and wheals were the chosen indexes because they are reported as frequent manifestations of exacerbations in allergic diseases and usually used in such studies9,12. Parameters commonly employed to evaluate rhinitis were used during the challenge10. Clinical examination of the chest commonly discloses signals of obstructive problems with air flow, especially with sequential PEFR measurements4,13. Potential effects of drugs commonly used to alleviate symptoms upon the test results were taken in consideration. Antihistamines and inhaled, short-acting bronchodilator aerosol were allowed when necessary. Inhaled steroids in intranasal and oral forms were accepted as prophylactic, associated or not with long acting bronchodilator aerosol. As a matter of fact, interrupting symptomatic drugs in subjects suffering from atopic diseases before challenges has been pointed out as a common bias7,13,14. Despite these considerations, no subjects were in regular use of antihistamines in the last six days prior to the first challenge. They were kept without drugs, since patients suffering from urticaria were asymptomatic by the time of the study. Nine subjects were on regular use steroids (6 intranasal and 3 orally inhaled) with two of them also using long-acting bronchodilator. An overview of the results showed that the responses to the evaluated parameters were within those expected, indicating reproducibility of the tests.
Conditions like the one of the patient who presented with urticaria at baseline in the second visit (placebo challenge) are frequent in challenges where a drug or a food is tested. Only anecdotal observations have pointed to these transient reactions as being associated with this drug1,9,15.
Despite the finding of a statistically significant difference between baseline values and the last evaluation in the treatment group specifically regarding cutaneous scoring, the magnitude of the reactions were very low, seating far below the mild level. For this reason, these findings were viewed as clinically irrelevant. More importantly, no differences were observed between placebo and drug treatments. Our results point to an absence of specific action of the yellow dye on the clinical atopic syndromes in the studied subjects.
Studies with conflicting results have appeared since the initial suggestions that yellow dyes participate in exacerbations of atopic diseases. These observations led to a frequently unacceptable use of wasting exclusion diets especially in patients suffering from urticaria and asthma, a practice which is not scientifically based.
Our findings are in agreement with the well conducted studies which minimised the importance of the yellow dye as a trigger of atopic diseases 7,12,13. Furthermore, in a Cochrane systematic review, routine tartrazine exclusion for asthmatic atopic patients is not recommended for individuals other than the ones with proven sensitivity5.
The relevance of the subject of our study, additives as triggers of atopic diseases, has been controversial16, and the concern still persists. In Brazil, for instance, ANVISA, similarly to the Code of Federal Regulations in United States, states that foods which contain FD&C Yellow No. 5 shall specifically declare its presence in the list of ingredients17,18. Regarding medicines, the authority demands the following warning statement: “This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity”19,20.
In spite of our limited sample, the statistical analyses could compare the clinical manifestations during oral provocation tests between tartrazine and placebo. A larger sample would be important to detect minor differences between the two groups. Nevertheless, our data, collected under an appropriate double-blind and cross-over study design, are in accordance with other well designed trials described in the literature7,12,13. In conclusion, we observed that by means of a double-blind placebo controlled cross-over challenge, tartrazine was no more likely than placebo to cause adverse reactions in a group of 26 Brazilian atopic adults with asthma, allergic rhinitis, urticaria or presenting allergic or pseudo allergic reactions to ASA or NSAIDs.
Conflict of interestThe authors have no conflict of interest to declare.