Glutaraldehyde-modified natural allergen extracts show significant reduction in the IgE-binding capacity and proteolytic activity. This allows the administration of higher doses in a shorter period of time, and to mix different allergen extracts.
ObjectiveEvaluate the safety of different concentrations and mixtures of glutaraldehyde-modified allergen extracts in a large group of paediatric and adult patients undergoing specific immunotherapy treatment.
Materials and methods1855 patients (1156 adults and 699 children), suffering from rhinoconjunctivitis and/or asthma, participated in an observational multicentre cohort study, to evaluate the safety of immunotherapy using vaccines containing modified allergen extracts. Patients were monosensitised, or polysensitised, and received a therapeutic vaccine containing polymerised allergen extracts adsorbed onto aluminium hydroxide. Safety was assessed by recording all side reactions related to immunotherapy.
ResultsThe clinically relevant local reactions totalled 120, (90 immediate and 30 delayed) (1.02% of injections). Of them, 31 (0.26% of injections) occurred in children (26 immediate and 5 delayed) and 89 (0.76% of injections) in adults (64 immediate and 25 delayed). There were 38 systemic reactions. Eleven reactions were immediate (9 of grade 1 and 2 of grade 2) and 27 delayed (22 of grade 1 and 5 of grade 2). There were seven grade 2 systemic reactions (0.06% of the injections). No differences (P>0.05) in the number of reactions were observed between adults and children and between treatments were found in systemic reactions. All systemic reactions were mild and resolved spontaneously without the need of medication.
ConclusionSpecific immunotherapy using natural modified allergen vaccines is safe to treat allergic patients, even at higher doses and in mixtures of unrelated allergen extracts. The percentage of adverse reactions detected is lower than those reported in the literature with native unmodified allergen extracts.
The efficacy and effectiveness of allergen specific immunotherapy (SIT) has been demonstrated in several double-blind placebo-controlled trials (DBPC) and in open studies.1–5 SIT has been accepted in consensus documents and position papers to be the only aetiological treatment of allergic diseases caused by inhalant allergens and hymenopter venoms,6–10 always considering that it is effective when appropriate doses of allergens are administered.3–9,11 The main concern related to this treatment is safety, which has been recognised since early studies12,13 and has been globally evaluated and reviewed.14–16 It has also been specifically evaluated in prospective studies,17–23 assessing the safety of allergen vaccines from a single, or various, manufacturers in large groups of patients.
The vast majority of side effects related to SIT with allergen extracts are mainly due to the intrinsic properties of the allergens (IgE-binding epitopes) and the susceptibility of the allergic patient. These are predictable but they are not necessarily acceptable. Severe side-effects may be acceptable if the degree of benefit is large and the risk of side-effects is minimised and treated promptly and effectively.12 In general, the clinical indications for immunotherapy for allergic rhinitis and asthma are similar for adults and children.11
An important issue is the increasing evidence that polysensitisation to allergically unrelated allergens is more prevalent than monosensitisation.24–26 The treatment of these patients with immunotherapy is a matter of debate.27 Two possibilities may arise: the use of a mixture of the relevant allergen extracts to which the patient is allergic,11 or the use of a single clinically relevant allergen.9 Single-allergen immunotherapy refers to a single extract, or to extracts containing several allergenically close related allergens, such as a grass-pollen mixture, or Dermatophagoides pteronyssinus and D. farinae. Multi-allergen immunotherapy is the use of allergen mixtures with little, or no allergenic cross-reactivity,24 such as Olea europaea and grass pollens, Cupressus spp. with other tree species, such as Platanus hybrida, grass mixtures with Salsola kali, or Parietaria Judaica, or D. pteronyssinus and Blomia tropicalis.
Consideration of the following basic principles is necessary when mixing allergen extracts: (1) cross reactivity of allergens, (2) appropriate dose of each constituent and (3) enzymatic degradation of allergens.11 In the case of multi-allergen immunotherapy, the current procedure is the mixture of the allergen extracts, obtaining a final preparation in which each extract is diluted by a factor which is the number of extracts (i.e. 50% and 50% in the case of two allergens, or 33, 33 and 33% in the cases of three allergens). Therefore, the effective dose of each constituent is decreased.11 The consideration regarding the maintenance of the appropriate dose in a mixture has a great impact on the issue of safety, because the maintenance of these doses yields a final preparation in which the final allergenic activity is the sum of the activity of each component. This approach, that could be dangerous using native allergen extracts, could be achieved using allergoids.
The modification of natural allergens with glutaraldehyde28,29 which produces extracts with decreased IgE-binding capacity has proven to be safe and clinically efficacious,30–37 allowing for the administration of high doses in a short period of time.38 Furthermore, this modification significantly reduces the proteolytic activity of some native allergen extracts, such as those derived from mites,39 allowing the mixture with pollen extracts.
The objective of this study was to evaluate the safety of therapeutic vaccines containing modified allergen extracts in a representative cohort of patients and to compare the safety profile regarding the age and the dose of allergen extract administered.
Safety was graded following the criteria of the European Academy of Allergy and Clinical Immunology (EAACI).8
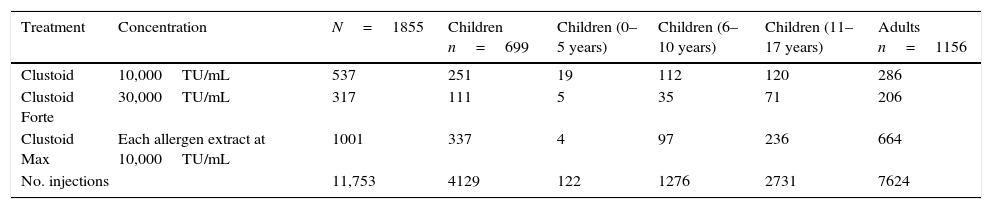
Materials and methodsPatient populationThe data evaluated were from the medical records of a cohort of 1855 patients (1034 male, 821 female). From these, 699 patients were children (435 males and 264 females) from 0 to 17 years with a mean age of 10.18 years (95% confidence interval 6.7–13.66) and 1156 patients were adults (599 males and 557 females) with a mean age of 34.59 years old (95% confidence interval 23.33–45.85). The epidemiological data of these patients are shown in Table 1. All patients were treated under real life clinical conditions in 48 Clinics/Allergy Services. The age distribution of the patients was: 699 (37.7%) were younger than 18 years. From these, 0.05% (one patient) was 10 months old, 14.6% were between 2 and 10 years and 23% were between 12 and 18 years. Adults were 1156 (62.3%) (Table 1). Patients were mono or polysensitised to mites (Dermatophagoides pteronyssinus, D. farinae, Blomia tropicalis), pollens (grasses, Olea europaea, Parietaria judaica, Cupressus arizonica, Salsola kali, Artemisia vulgaris, Plantago lanceolata and/or Chenopodium album) and/or danders (cat and/or dog). All patients met the criteria for receiving immunotherapy described in the EAACI and WHO Position Papers on allergen immunotherapy.40,41 All patients were diagnosed as having rhinoconjunctivitis (RC) (n=1002) or rhinoconjunctivitis and asthma (RC-A) (n=883).
Immunotherapy preparationEach patient received an individualised commercial therapeutic vaccine (Clustoid®, Inmunotek, SL, Alcalá de Henares, Spain). Three different preparations were supplied according to the individual medical prescription: Clustoid®, Clustoid® Max or Clustoid® Forte. The characteristics of each preparation are specified in Table 2. All of them contained polymerised allergen extracts prepared as previously described.42 Each treatment contained vials of a single concentration of the modified extract/s labelled in Therapeutic Units (TU). One TU of the modified extract has the same protein amount as the protein of one Biological Unit (BU) of the corresponding native extract standardised according to the Nordic Guidelines.43
Concentration and frequency of the treatments administered.
| Treatment | Concentration | N=1855 | Children n=699 | Children (0–5 years) | Children (6–10 years) | Children (11–17 years) | Adults n=1156 |
|---|---|---|---|---|---|---|---|
| Clustoid | 10,000TU/mL | 537 | 251 | 19 | 112 | 120 | 286 |
| Clustoid Forte | 30,000TU/mL | 317 | 111 | 5 | 35 | 71 | 206 |
| Clustoid Max | Each allergen extract at 10,000TU/mL | 1001 | 337 | 4 | 97 | 236 | 664 |
| No. injections | 11,753 | 4129 | 122 | 1276 | 2731 | 7624 | |
Major allergen content was determined in the corresponding native preparation before modification and expressed in mcg/mL. The contents in mite extracts was determined by ELISA using monoclonal antibodies (Indoor Biotechnologies Ltd., Charlottesville, VA, USA) and in pollen extracts by means of scanning densitometry.44 The contents in these major allergens in the native extract needed to produce 10,000 TU of the modified preparation were 4 and 2 mcg of group 1 and group 2 of Dermatophagoides, respectively. In pollen, the figures were 24 mcg of Ole e 1 (O. europaea), and 25 mcg of Phl p1+Phl p 5 in grasses.
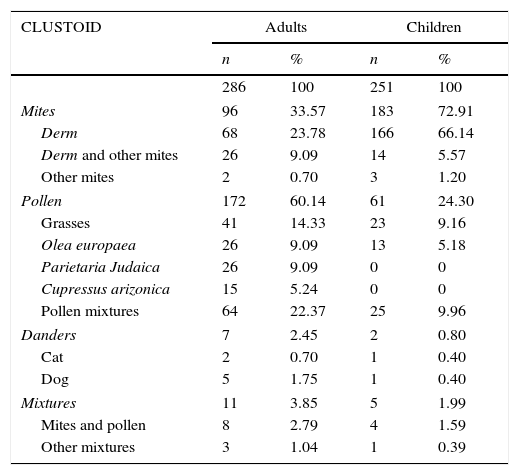
The composition of the treatments is described in Table 3.
Composition of the vaccines administered to the adult and children patients.
| CLUSTOID | Adults | Children | ||
|---|---|---|---|---|
| n | % | n | % | |
| 286 | 100 | 251 | 100 | |
| Mites | 96 | 33.57 | 183 | 72.91 |
| Derm | 68 | 23.78 | 166 | 66.14 |
| Derm and other mites | 26 | 9.09 | 14 | 5.57 |
| Other mites | 2 | 0.70 | 3 | 1.20 |
| Pollen | 172 | 60.14 | 61 | 24.30 |
| Grasses | 41 | 14.33 | 23 | 9.16 |
| Olea europaea | 26 | 9.09 | 13 | 5.18 |
| Parietaria Judaica | 26 | 9.09 | 0 | 0 |
| Cupressus arizonica | 15 | 5.24 | 0 | 0 |
| Pollen mixtures | 64 | 22.37 | 25 | 9.96 |
| Danders | 7 | 2.45 | 2 | 0.80 |
| Cat | 2 | 0.70 | 1 | 0.40 |
| Dog | 5 | 1.75 | 1 | 0.40 |
| Mixtures | 11 | 3.85 | 5 | 1.99 |
| Mites and pollen | 8 | 2.79 | 4 | 1.59 |
| Other mixtures | 3 | 1.04 | 1 | 0.39 |
| CLUSTOID FORTE | Adults | Children | ||
|---|---|---|---|---|
| n | % | n | % | |
| 206 | 100 | 111 | 100 | |
| Mites | 144 | 69.90 | 84 | 75.68 |
| Derm | 136 | 66.02 | 84 | 75.68 |
| Derm and other mites | 8 | 3.88 | ||
| Pollen | 61 | 29.61 | 27 | 24.32 |
| Grasses | 58 | 28.16 | 23 | 20.72 |
| Other pollen | 3 | 1.45 | 4 | 3.60 |
| Mixtures | 1 | 0.49 | 0 | 0 |
| Grasses and Derm | 1 | 0.49 | 0 | 0 |
| CLUSTOID MAX | Adults | Children | ||
|---|---|---|---|---|
| n | % | n | % | |
| 664 | 100 | 337 | 100 | |
| Mites | 173 | 26.05 | 99 | 29.38 |
| Derm and other mites | 33 | 4.96 | 16 | 4.75 |
| Other mites | 1 | 0.15 | 0 | 0 |
| Derm and grasses | 39 | 5.87 | 22 | 6.53 |
| Derm, grasses and other pollen | 26 | 3.91 | 10 | 2.97 |
| Derm and other pollen | 53 | 7.98 | 39 | 11.5 |
| Derm, mites, cat and/or pollen | 18 | 2.71 | 3 | 0.89 |
| Derm, mites and pollen | 1 | 0.15 | 2 | 0.59 |
| Other mites and pollen | 2 | 0.30 | 1 | 0.30 |
| Pollen | 441 | 66.42 | 238 | 70.62 |
| Grasses and other pollen | 378 | 56.93 | 225 | 66.76 |
| Grasses, cat dander and/or other pollen | 13 | 1.96 | 3 | 0.89 |
| Other mixtures | 50 | 7.53 | 10 | 2.97 |
Abbreviation: Derm means Dermatophagoides pteronyssinus and Dermatophagoides farinae.
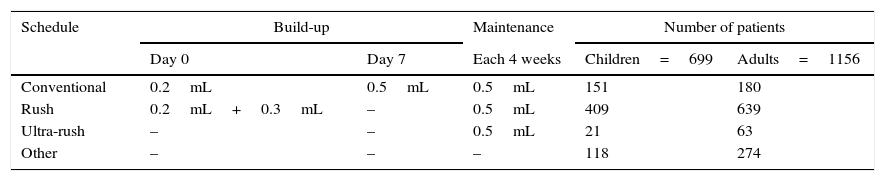
Different treatment schedules were used by the clinicians, with the most common being: the conventional, rush and ultra-rush. In conventional schedules, (n=331; 151 children and 180 adults) the patient received an injection of 0.2mL and after one-week 0.5mL. In rush schedule (n=1048; 409 children and 639 adults) the first dose of 0.2mL was injected in one arm and 30min later 0.3mL in the other arm. In ultra-rush schedule (n=84; 21 children and 63 adults) the first injection was the maintenance dose of 0.5mL (Table 4). In all schedules, the maintenance dose was 0.5mL monthly. Pre-treatment with drugs (antihistamines or corticosteroids), in order to prevent adverse reactions, was not used. All injections were administered subcutaneously on the extensor surfaces of the upper arms using an insulin syringe with a 26 gauge ½ inch non-removable needle. Following the recommendations of the European Academy of Allergy and Clinical Immunology (EAACI),40 all patients had a 30minutes waiting period after each injection. Immunotherapy treatments including pollen extract were administered without interruption, or reduction of the planned dose during the corresponding pollen season.
Ethics, design and sample size calculationThe study was classified as Observational Postauthorization study (EPA-OD) by the Spanish Health Authorities (Agencia Española de Medicamentos y Productos Sanitarios) and was evaluated by the Ethics Committee of the “Comunidad Autonómica de Madrid”. It was conducted in 48 centres in Spain involving 57 specialists in allergy for the safety evaluation of different treatments containing polymerised allergen extracts (Clustoid®, Clustoid® Forte and Clustoid® Max, Inmunotek, Alcalá de Henares, Spain). Table 1 shows the characteristics of each preparation. The sample size was calculated based on the results obtained by Gastaminza.17 The rate of systemic reactions (grades 2, 3 and 4) was 0.27% per injection. We considered that the rate of systemic reactions using these polymerised allergen extracts would be reduced by a factor of three. Therefore, the number of injections to be evaluated, for a relative risk of 0.35 and an error protection of 7.84,45 was 9655. Considering that each patient would receive a mean of six injections, the number of patients to obtain this data was a minimum of 1650 subjects.
Safety assessmentThe adverse reactions based on the intrinsic properties of the allergen extracts were categorised as immediate when the onset was during the first 30minutes after the administration and delayed when the onset was afterwards.8 Local reactions were quantified measuring the diameter of the erythema of the reaction. Immediate reactions with a diameter smaller than 5cm and delayed reactions smaller than 10cm were considered as clinically irrelevant.46 The systemic reactions were graded according to the classification of the EAACI Position Paper on immunotherapy.8 The grading was: grade 0: No symptoms or non-specific symptoms; grade 1: Mild systemic reactions. Symptoms: Localised urticaria, rhinitis or mild asthma (Peak Flow<20% decrease from baseline); grade 2: Moderate systemic reactions. Symptoms: Slow onset (>15min) of generalised urticaria and/or moderate asthma (PF<40% decrease from baseline); grade 3: Severe (non-life-threatening) systemic reactions. Symptoms: Rapid onset (<15min) of generalised urticaria, angio-oedema, or severe asthma (PF>40% decrease from baseline), and grade 4: Anaphylactic shock. Symptoms: Immediate evoked reaction of itching, flushing, erythema, generalised urticaria, stridor (angio-oedema), immediate asthma, hypotension etc.
StatisticsFisher's exact test was used to evaluate the differences in the number of side reactions between children and adults, and the build-up and maintenance doses of different mixtures (mites, pollen and mites+pollen) and between the preparations containing different number of extracts.
ResultsVaccines and number of injectionsThe composition of the vaccines and schedules of administration in children and adults are shown in Tables 3 and 4, respectively. The number of injections administered was 11,753; 4129 (35.1%) corresponded to the children and 7624 (64.9%) to the adults. From these, 3292 (28%) corresponded to the build-up phase (1228 in children and 2064 in adults) and 8461 (72%) to the maintenance dose (2901 in children and 5560 in adults) (data not shown). Each patient received a mean of 6.33 injections (5.9 in children and 6.6 in adults).
Adverse reactionsAll reactions were due to the intrinsic properties of the allergen extracts and, therefore, expected. There were no records related to unexpected adverse reactions, such as of toxic or infectious origins.
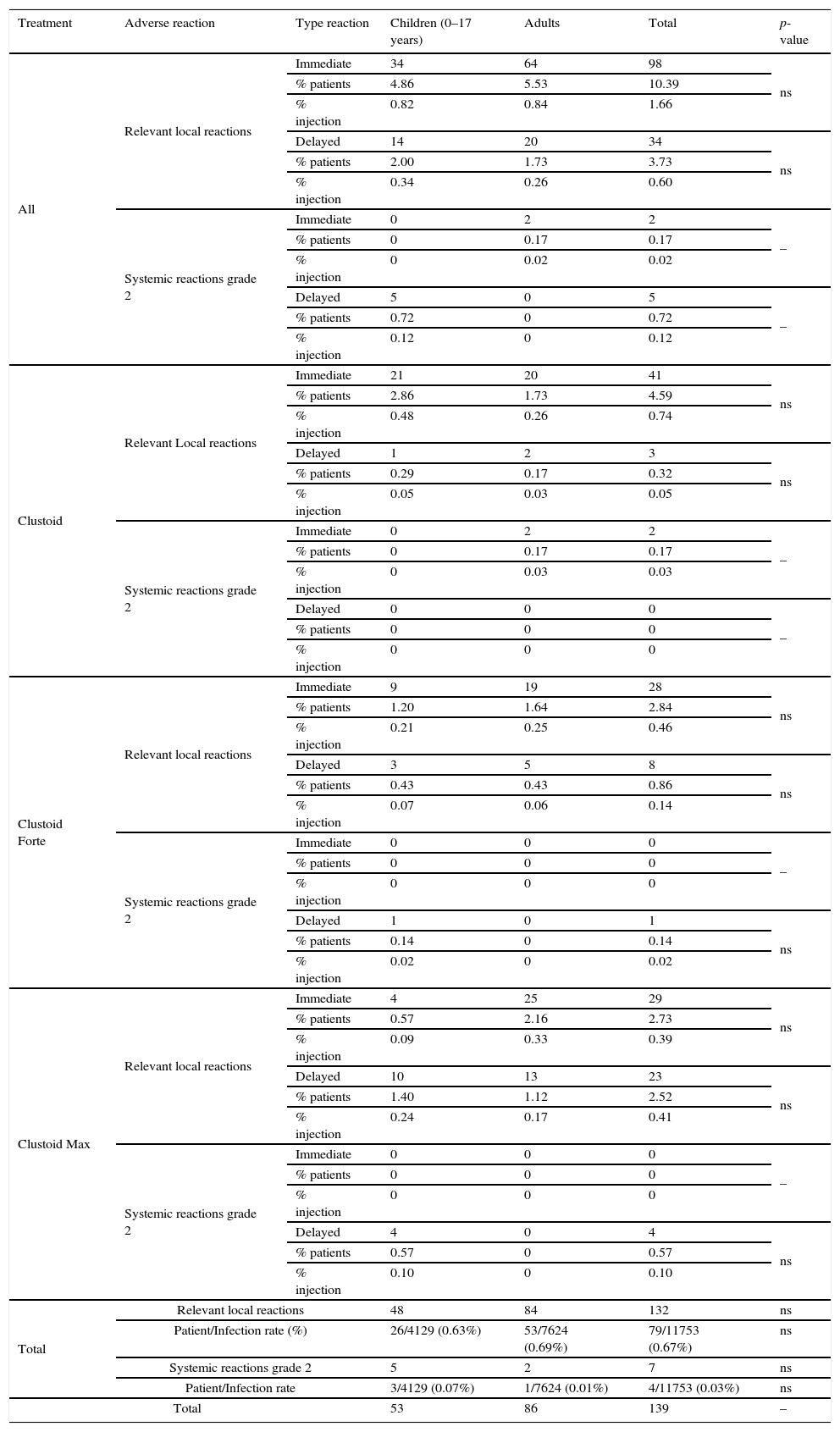
A total of 132, (98 immediate and 34 delayed) relevant local reactions (1 per 14 patients, and 1 per 89 injections) were recorded. Of them, 48 (0.40% of injections) occurred in children (34 immediate and 14 delayed) and 84 (0.71% of injections) in adults (64 immediate and 20 delayed). There were significant differences between Clustoid® Max and both, Clustoid® and Clustoid® Forte, regarding relevant local reactions (P=0.017 and P=0.004 respectively, Fisher exact text; data not shown).
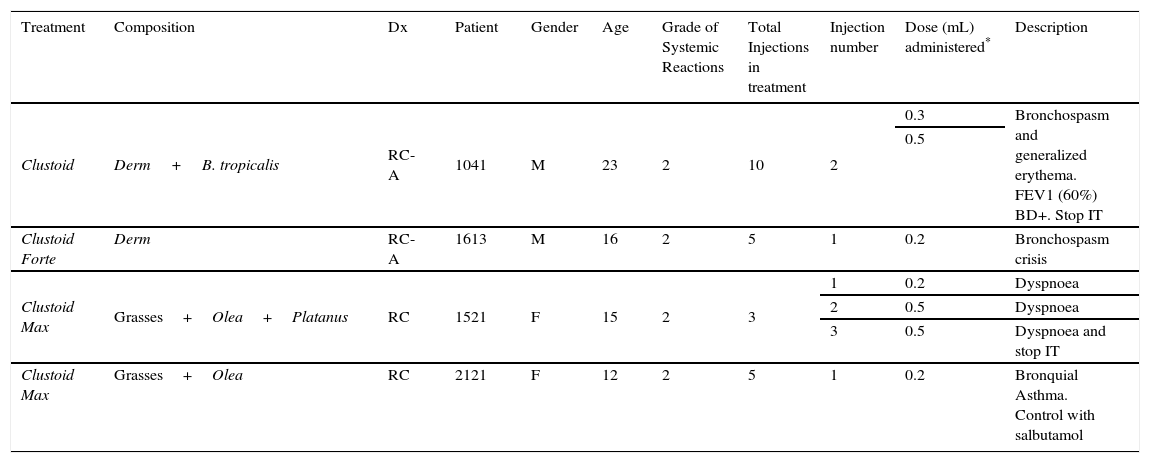
There were 38 systemic reactions in 1855 patients (14 in children and 24 in adults). 11 reactions (8 in adults and 3 in children) were immediate (9 of grade 1 and 2 of grade 2) and 27 delayed (11 in children and 16 in adults, 22 of grade 1 and 5 grade 2) (data not shown). There were 7 systemic reactions of grade 2 (occurred in 0.06% of the injections). All systemic reactions were mild and resolved (Table 5). No differences between children and adults and between treatments were found in systemic reactions. Two patients withdrew from immunotherapy due to reactions (described in Table 6).
Adverse local and systemic relevant reactions by treatment in children and adults.
| Treatment | Adverse reaction | Type reaction | Children (0–17 years) | Adults | Total | p-value |
|---|---|---|---|---|---|---|
| All | Relevant local reactions | Immediate | 34 | 64 | 98 | ns |
| % patients | 4.86 | 5.53 | 10.39 | |||
| % injection | 0.82 | 0.84 | 1.66 | |||
| Delayed | 14 | 20 | 34 | ns | ||
| % patients | 2.00 | 1.73 | 3.73 | |||
| % injection | 0.34 | 0.26 | 0.60 | |||
| Systemic reactions grade 2 | Immediate | 0 | 2 | 2 | – | |
| % patients | 0 | 0.17 | 0.17 | |||
| % injection | 0 | 0.02 | 0.02 | |||
| Delayed | 5 | 0 | 5 | – | ||
| % patients | 0.72 | 0 | 0.72 | |||
| % injection | 0.12 | 0 | 0.12 | |||
| Clustoid | Relevant Local reactions | Immediate | 21 | 20 | 41 | ns |
| % patients | 2.86 | 1.73 | 4.59 | |||
| % injection | 0.48 | 0.26 | 0.74 | |||
| Delayed | 1 | 2 | 3 | ns | ||
| % patients | 0.29 | 0.17 | 0.32 | |||
| % injection | 0.05 | 0.03 | 0.05 | |||
| Systemic reactions grade 2 | Immediate | 0 | 2 | 2 | – | |
| % patients | 0 | 0.17 | 0.17 | |||
| % injection | 0 | 0.03 | 0.03 | |||
| Delayed | 0 | 0 | 0 | – | ||
| % patients | 0 | 0 | 0 | |||
| % injection | 0 | 0 | 0 | |||
| Clustoid Forte | Relevant local reactions | Immediate | 9 | 19 | 28 | ns |
| % patients | 1.20 | 1.64 | 2.84 | |||
| % injection | 0.21 | 0.25 | 0.46 | |||
| Delayed | 3 | 5 | 8 | ns | ||
| % patients | 0.43 | 0.43 | 0.86 | |||
| % injection | 0.07 | 0.06 | 0.14 | |||
| Systemic reactions grade 2 | Immediate | 0 | 0 | 0 | – | |
| % patients | 0 | 0 | 0 | |||
| % injection | 0 | 0 | 0 | |||
| Delayed | 1 | 0 | 1 | ns | ||
| % patients | 0.14 | 0 | 0.14 | |||
| % injection | 0.02 | 0 | 0.02 | |||
| Clustoid Max | Relevant local reactions | Immediate | 4 | 25 | 29 | ns |
| % patients | 0.57 | 2.16 | 2.73 | |||
| % injection | 0.09 | 0.33 | 0.39 | |||
| Delayed | 10 | 13 | 23 | ns | ||
| % patients | 1.40 | 1.12 | 2.52 | |||
| % injection | 0.24 | 0.17 | 0.41 | |||
| Systemic reactions grade 2 | Immediate | 0 | 0 | 0 | – | |
| % patients | 0 | 0 | 0 | |||
| % injection | 0 | 0 | 0 | |||
| Delayed | 4 | 0 | 4 | ns | ||
| % patients | 0.57 | 0 | 0.57 | |||
| % injection | 0.10 | 0 | 0.10 | |||
| Total | Relevant local reactions | 48 | 84 | 132 | ns | |
| Patient/Infection rate (%) | 26/4129 (0.63%) | 53/7624 (0.69%) | 79/11753 (0.67%) | ns | ||
| Systemic reactions grade 2 | 5 | 2 | 7 | ns | ||
| Patient/Infection rate | 3/4129 (0.07%) | 1/7624 (0.01%) | 4/11753 (0.03%) | ns | ||
| Total | 53 | 86 | 139 | – | ||
Abbreviation: ns, not significant.
Description of systemic reactions.
| Treatment | Composition | Dx | Patient | Gender | Age | Grade of Systemic Reactions | Total Injections in treatment | Injection number | Dose (mL) administered* | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| Clustoid | Derm+B. tropicalis | RC-A | 1041 | M | 23 | 2 | 10 | 2 | 0.3 | Bronchospasm and generalized erythema. FEV1 (60%) BD+. Stop IT |
| 0.5 | ||||||||||
| Clustoid Forte | Derm | RC-A | 1613 | M | 16 | 2 | 5 | 1 | 0.2 | Bronchospasm crisis |
| Clustoid Max | Grasses+Olea+Platanus | RC | 1521 | F | 15 | 2 | 3 | 1 | 0.2 | Dyspnoea |
| 2 | 0.5 | Dyspnoea | ||||||||
| 3 | 0.5 | Dyspnoea and stop IT | ||||||||
| Clustoid Max | Grasses+Olea | RC | 2121 | F | 12 | 2 | 5 | 1 | 0.2 | Bronquial Asthma. Control with salbutamol |
Abbreviation: Derm, Dermatophagoides pteronyssinus and Dermatophagoides farinae; Dx, diagnosis; RC, rhinoconjuntivitis; RC-A, rhinoconjuntivitis and asthma.
Retrospective data related to the administration of vaccines containing polymerised allergen extracts, from the medical records of a cohort of 1855 patients, was analysed in order to evaluate the safety of these preparations. A large number of centres (n=48) and physicians (n=57) were included in order to be more representative of real life conditions and to avoid the bias of a single, or few, physicians administering the treatment. All adverse reactions were of allergic nature, expected during the use of specific immunotherapy. In the present study, we detected that 1.12% of the total injections caused local reactions and that 0.06% caused a systemic reaction of grade 2. No grade 3 and 4 systemic reactions were reported.
Maintenance dose was reached the first day using rush schedule, or in one week with just two injections.
We observed that specific immunotherapy (AIT) using modified allergen vaccines with different schedules is safe in both children and adults. In this study we have found a low rate of adverse reactions to AIT in Clustoid®, Clustoid® Forte and Clustoid® Max treatments in both of them. Significant differences between children and adults were not observed with the use of Clustoid® Forte, Clustoid® Forte and Clustoid® Max treatments related to relevant local reactions. These data suggest that these treatments appear to be better-tolerated in children than in adults even at higher concentrations like in Clustoid® Forte (30,000TU/mL). To confirm these data will need studies with a larger number of patients.
With the use of unmodified standardised allergen extracts adsorbed to alum, Gastaminza et al.17 provided data from 1212 patients in which SIT was administered in a single immunotherapy unit. These patients were diagnosed of allergic asthma and/or rhinoconjunctivitis, or hymenoptera venom anaphylaxis, and received a total of 29,754 injections. Seventy-nine systemic reactions (0.3% per injection) occurred in 2.9% of the patients receiving mite extracts, 7.5% of patients with pollen extracts and 6.7% in the case of hymenoptera venoms. No withdrawals were reported.
In a multi-centre study, Moreno et al.18 evaluated 488 patients treated with biologically standardised allergen extracts for respiratory allergies; 433 patients completed the study and four were asked to abandon by the physicians. In three cases, the abandonment was suggested due to recurrent reactions and in one due to a single reaction. Four more patients left the study by their own decision due to local reactions during the build-up phase. The total of injections administered was 17,526 and the number of systemic reactions was 50 (0.3% of the injections): 30 of grade 2 and 21 of grade 3. In this study, the investigators detected more systemic reactions in patients receiving mite extracts.
Winther et al.19 evaluated the side-effects of SIT of a single manufacturer in a multi-centre study involving 1038 patients, treated with extracts of mite, pollen, dog or cat, or with wasp and bee venoms. The total number of injections was 23,047. Forty-one patients (4%) stopped the treatment due to complications (local or systemic reactions, or worsening of allergic symptoms). Twenty-three per cent of the patients treated with one allergen extract did not reach the maximum dose, and 29% when the treatment was with more than one allergen extract. A total of 582 side-effects were reported in 341 patients (2.5% of the injections). Seventy-eight per cent of these reactions were of grade 2, 20% of grade 3, and 1% (eight reactions) of grade 4.
Tabar et al.21 analysed 419 patients observing that 4.8% of the patients experienced a systemic reaction (0.4% of the injections), being these reactions mainly in patients with asthma and sensitised to D. pteronyssinus. In another study,20 239 patients were evaluated in a double-blind placebo-controlled study using an allergen extract of D. petronyssinus from a single manufacturer administered using two types of build-up phases (conventional and cluster). During the incremental phase, there were 19 adverse reactions in 15 patients receiving the allergen extract; eight reactions (0.2% per injection) were systemic (three immediate and five delayed). All reactions were of grade 2. During the maintenance phase, nine delayed adverse reactions (0.5% per injection) were reported in eight patients. Two reactions were local and seven systemic (one with non-specific symptoms and six with asthma). As a consequence, the dose schedule for these patients was modified. Machín et al.22 evaluated the safety in 339 patients carefully treated in an immunotherapy unit, observing 21 systemic reactions in 14 patients (4.1% of the patients, 0.4% of the injections). In other double-blinded, placebo-controlled studies we can observed that pre-seasonal SCIT in the elderly is safe and efficacious comparable to studies in younger patients, although SCIT were safe in both.47
The tolerability of modified extracts was compared in double-blind studies with the corresponding unmodified preparations.36,37 Doses 10 times higher than those used in the present study were achieved, and no adverse events were reported with the doses used in normal clinical conditions, confirming the wide therapeutic margin for this chemically-modified allergen vaccine and the safer profile than regular vaccines based on native unmodified extracts.
Although this study was not designed to compare SCIT with the sublingual route of administration, the rate of side reactions described in this study is lower than that described in some studies using sublingual immunotherapy. The safety of sublingual immunotherapy, using drops or tablets, has been reviewed in a study including eight double-blind placebo-controlled (DBPC) trials.48 A total of 690 patients (347 active and 343 placebo) were studied; 145 adverse events were reported in the active and 79 in the placebo group (p<0.01), with a higher incidence of adverse events in the mouth (18% and 4% of the patients in the active and placebo groups, respectively) and in the gastro-intestinal tract (14% and 4% of the patients in the active and placebo groups, respectively). Two laryngeal oedemas were reported in patients receiving the active treatment (0.6% of the patients).
In DBPC studies using sublingual tablets of Phleum pratense49 and House Dust Mite,50 there were a lot of non-explained withdrawals due to adverse events in the groups who received the active tablets. Finally, in a large study using tablets,51 855 patients were included and 451 (53%) had adverse events, judged by the investigators as probably, or possibly, related to the treatment; 26 participants (3%) withdrew due to adverse events. Other studies with sublingual immunotherapy tablets describe a high percentage of adverse reactions with grasses and mites.52,53
Two safety studies23,38 using modified allergen extracts of another manufacturer involved 1834 patients. One (1068 patients)38 was regarding the safety of the build-up phase of rush schedule and the other (766 patients) build-up and maintenance. The incidence of relevant local and systemic reactions was low, below 1%, and all systemic reactions were mild and resolved spontaneously without the need for medication. There is some mention of oral immunotherapy with modified allergen extracts and safety. No severe adverse events effects were observed in short-term study.54
The possibility of adverse reactions of infectious origin has been suggested because of the use of multidose vials. These vials are sterile and contain phenol to avoid further contamination when in use. In this study, no reactions due to infection were recorded. This is in line with previous publications by Balekian et al.55 and Lay et al.,56 who evaluated the presence of adverse events of infectious origin after the administration of more than 130,000 injections in more than 3000 patients and more than 26,000 injections in 272 patients, respectively.
Considering the results presented in this study and in the context of the data available in the literature, we can conclude that the SC administration of these modified allergen extracts is safer than the use of unmodified allergen vaccines in all groups of patients. Furthermore, the available published data suggests that the safety profile of these modified allergen vaccines administered subcutaneously could be better than that observed with the sublingual administration of allergen extracts.
In conclusion, specific immunotherapy using modified natural allergen vaccines is safe to treat allergic patients, even at higher doses and in mixtures of unrelated allergen extracts without dilution in the final presentation.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors declare the following, real or perceived conflicts of interest: The authors work in Inmunotek SL.
The authors wish to thank the collaboration of all the participants in the study, including prescribing doctors and nurses. This study was sponsored by Inmunotek, S.L.