The effects of rosmarinic acid (RA) on immunological and inflammatory mediator levels in bronchoalveolar lavage fluid (BALF) as well as lung pathological changes in asthmatic rats were investigated.
MethodsThe levels of IFN-γ, IL-4, IFN-γ/IL-4 ratio, IgE, PLA2, and total protein (TP) in BALF and pathological changes in the lung were evaluated in control group (C), asthma group (sensitized to ovalbumin) (A), asthma groups treated with RA and dexamethasone.
ResultsCompared to the control group, asthmatic rats showed increased levels of IL-4, IgE, PLA2, and TP as well as all pathological scores with decreased levels of IFN-γ and IFN-γ/IL-4 ratio (P<0.05 to P<0.001). The levels of IL-4, IgE, PLA2, and TP significantly reduced in groups treated with all concentrations of RA compared to asthma group (P<0.001 for all cases). IFN-γ was significantly decreased in groups treated with two lower concentrations of RA but IFN-γ/IL-4 ratio was increased in groups treated with two higher concentrations of RA compared to asthma group (P<0.05 to P<0.001). Treatment with all doses of RA led to significant improvement in pathological scores in asthmatic animals (P<0.05 to P<0.001). Most measured parameters were also significantly improved in dexamethasone-treated animals (P<0.01 to P<0.001) but IFN-γ/IL-4 ratio and the scores of interstitial fibrosis, bleeding and epithelial damage did not change in this group.
ConclusionThe results indicated a preventive effect for RA on immunological and inflammatory mediators as well as lung pathological changes in asthmatic rats which were comparable or even more potent than that of dexamethasone.
Asthma is a chronic inflammatory disease of airways that involves inflammatory cells and mediators resulting in an inflammatory response, and asthma is characterized by airway inflammation and remodeling.1 Inflammatory cells such as T lymphocytes, eosinophils and mast cells play a vital role in the pathogenesis of immune-mediated diseases like asthma.2 In asthma, Th2 lymphocytes secrete IL-4, IL-5 and IL-13 which initiate allergic responses by increasing eosinophils infiltration and IgE production.3 IFN-γ which is secreted by Th1 cells, demonstrates inhibitory effects on Th2 cell differentiation.4 Therefore, Th1/Th2 imbalance due to increased Th2 activity leads to allergic and atopic diseases including asthma, atopic dermatitis, anaphylactic shock, and allergic rhinitis.5 In addition, previous studies have demonstrated the roles of phospholipase A2 (PLA2) and total protein in asthma.6,7 PLA2 has an important role in the production of eicosanoids and development of inflammation and airway hyperresponsiveness (AHR) in murine models of asthma.8 The possible roles of PLA2 in asthma include induction of the release of arachidonic acid and cytokines, generation of lysophospholipids, and degradation of surfactant along with its impact on immunological and inflammatory cells, such as dendritic cells, T-cells, and leukocytes.8 Increased PLA2 and total protein was also shown in BALF of ovalbumin (OVA)-sensitized rat.7 In asthmatic patients, increased serum level of IgE was reported.9 Circulating IgE activates mast cells and basophils and leads to the release of preformed mediators, such as histamine, prostaglandin and cysteinyl–leukotriene, which are associated with bronchoconstriction and plasma exudation.10
Inflammatory mediators, such as reactive oxygen species (ROS), histamine and products of arachidonic acid metabolism can induce airway remodeling such as the occurrence of goblet cell hyperplasia, enlarged submucosal mucus glands and vascular congestion, thickening of airway walls, infiltration of inflammatory cells, increases in smooth muscle mass, airway epithelial shedding, and the production of mucus plugs occluding medium and small bronchi.11 Structural changes in the airways lead to irreversible airflow obstruction and AHR in asthma.12
Rosmarinic acid (RA) is a naturally occurring phenolic compound which is found in several plants of the Lamiaceae family such as Ocimum basilicum, Rosmarinus officinalis, Origanum vulgare, Melissa officinalis and Salvia officinalis.13 These plants have been traditionally used for the treatment of airway diseases, such as asthma, allergic rhinitis, and otitis media.14 Different therapeutic effects have been described for RA such as antibacterial,15 anti-inflammatory16 and immunomodulatory actions17 and analgesic activities.18 The inhibitory effect of RA on airway inflammation and hyperresponsiveness was also reported in a murine model of asthma.19 Moreover, a considerable amount of evidence showed that ROS is responsible for the induction of inflammatory conditions such as asthma. In this regard, agents with antioxidant properties have attracted attention to be examined as potential therapeutics against conditions associated with inflammation.20,21
Given the antioxidant potential of RA, we were motivated to investigate the effects of RA on OVA-induced asthma. Therefore, in the present study, the effect of RA on IFN-γ, IL-4, IgE, PLA2 and total protein (TP) levels in BALF as well as its influence on lung pathological changes in asthmatic rats was evaluated.
Materials and methodsAnimalsThirty-six male Wistar rats weighing 200±20g were used in this study. The rats were kept in an animal house (in School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran) under standard conditions at 22±2°C with 12h/12h light/dark cycles and clean filtered air (Maximiser, Thorens Caging System Inc., Hazleton, PA, USA). Rats had free access to food and water ad libitum during the experimental period.
Experimental designAnimals were randomly divided into six groups (n=8 for RA-treated groups and n=6 for other groups) as follows:
- •
Control group (group C): Received intra-peritoneal (i.p.) and inhalation of normal saline
- •
Asthmatic group (group A): Received intra-peritoneal (i.p.) and inhalation of ovalbumin (OVA)
- •
Dexamethasone group (group A+D): Asthmatic rats treated with dexamethasone (1.25μg/mL)
- •
Rosmarinic acid groups (group A+RA): Asthmatic rats treated with RA 0.125, 0.250 and 0.500mg/mL
- •
Dexamethasone and RA were added to animals’ drinking water during the sensitization period. Each animal consumed almost 40mL water per day and this volume was not significantly different among different groups.
Animals were sensitized to OVA using the method described in our previous study.22 Briefly, rats were sensitized by three i.p. injections of 1mg/kg chicken egg albumin (OVA, grade V, 98% pure, Sigma, St. Louis, MO, USA) in 0.9% sterile saline containing 100mg Al(OH)3 as adjuvant, on days 1, 2 and 3 of the experiment. The animals were then exposed to an aerosol of 1% OVA with an air flow of 8 L/min produced by a DeVilbiss PulmoSonic nebulizer (DeVilbiss Health Care Ltd., Feltham, U.K.), on days 6, 9, 12, 15, 18 and 21 for 20min/day. Challenges were conducted in a 0.8m3 chamber, while the animals were breathing normally. Control animals were treated similarly but saline was used instead of OVA solution.
Preparation of BALF and measurement of the immunological and inflammatory mediatorsThe rats were anesthetized using ketamine hydrochloride (50mg/kg, i.p.) 24h after the last challenge (on the 22nd day). Chest was opened, lungs were dissected, and the left lung was lavaged five times with 1mL of saline (a total of 5mL). Then, the lavage sample was centrifuged at 2500g at 4°C for 10min. The supernatant was collected and immediately stored at −70°C until it was analyzed.7
Levels of PLA2, IFN-γ, IL-4 and IgE in BALF were measured using the enzyme-linked immune sorbent assay (ELISA) sandwich method according to the manufacturer's instructions (IFN-γ, IL-4 and IgE Rat ELISA Kit, Abcam were obtained from Cambridge, MA, USA and Rat Phospholipase A2 (PLA2) ELISA Kit was purchased from MyBioSource, San Diego, CA, USA). The TP level was photometrically determined using quantitative protein assay kit (Pars Azmon, Tehran, Iran) according to the manufacturer's protocol.
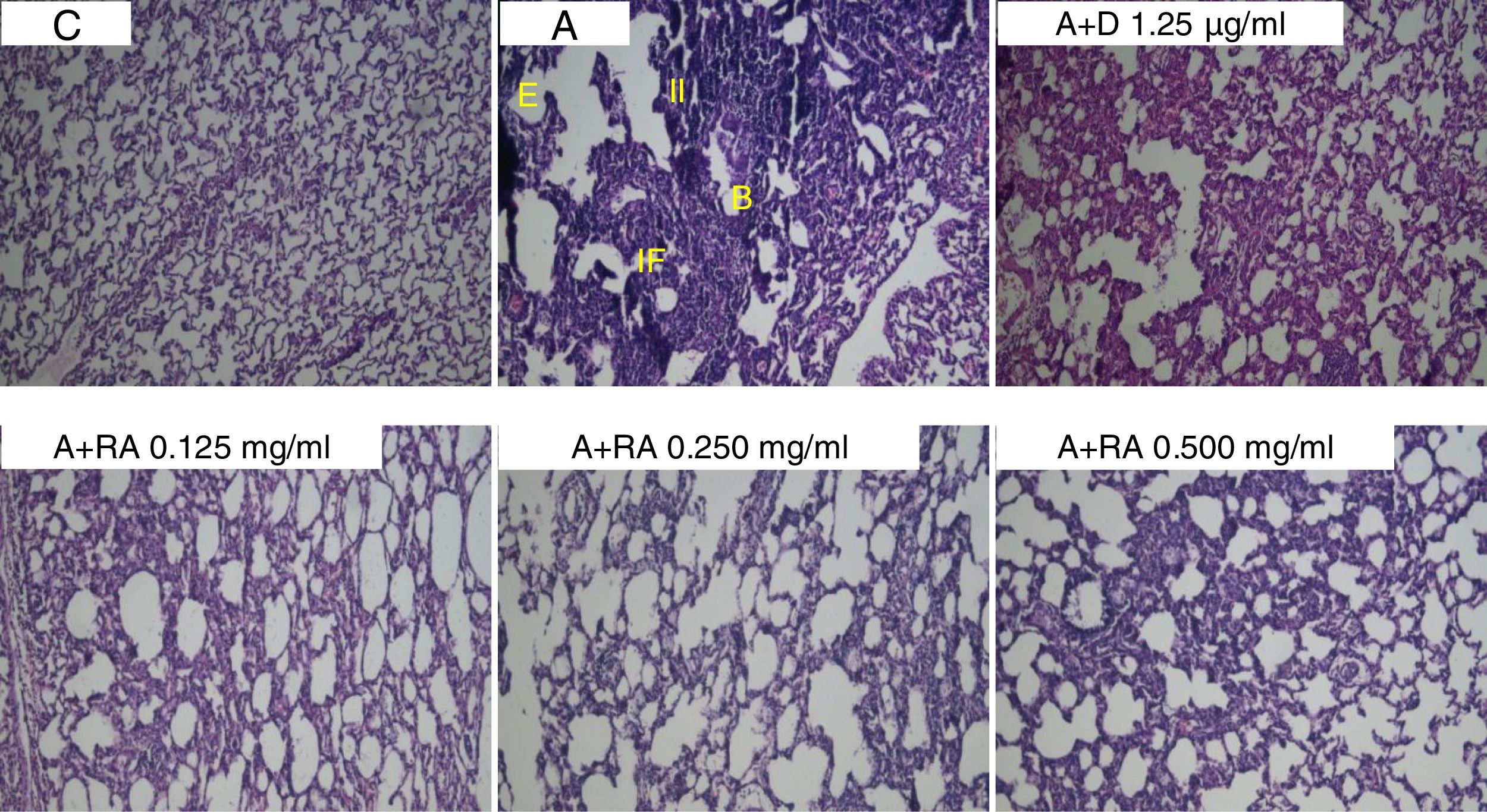
Pathological evaluationAs previously mentioned, rats were sacrificed, and lungs were dissected. The right lung was placed into 10% buffered formalin (Merck, Darmstadt, Germany). Seven days later, tissues were dried using Autotechnicon apparatus (Hisure, Zhejiang, China) and cleared by passing the tissues through ethanol 70–100% and xylol; finally, paraffin blocks were prepared. The specimens were cut into 4-μm slices and stained with hematoxylin and eosin (H&E). The tissues were then evaluated under a light microscope. The pathological features assessed in the lungs of control, asthmatic and treated groups included interstitial inflammation, interstitial fibrosis, bleeding, epithelial damage and emphysema. The pathological changes were scored according to previous studies23,24 as follows: (0) No pathological changes; (1) patchy changes and (2) severe changes.
Statistical analysisAll data were represented as mean±SEM. Normal distribution and equality of variances of the results were checked by Kolmogorov–Smirnov test. The sample size was chosen according to previous studies.19,22 Statistical comparisons were performed using one-way analysis of variance (ANOVA) with Tukey–Kramer as post hoc test. The results were considered statistically significant if P<0.05. InStat (GraphPad Software, Inc, La Jolla, USA) was used for data analysis.
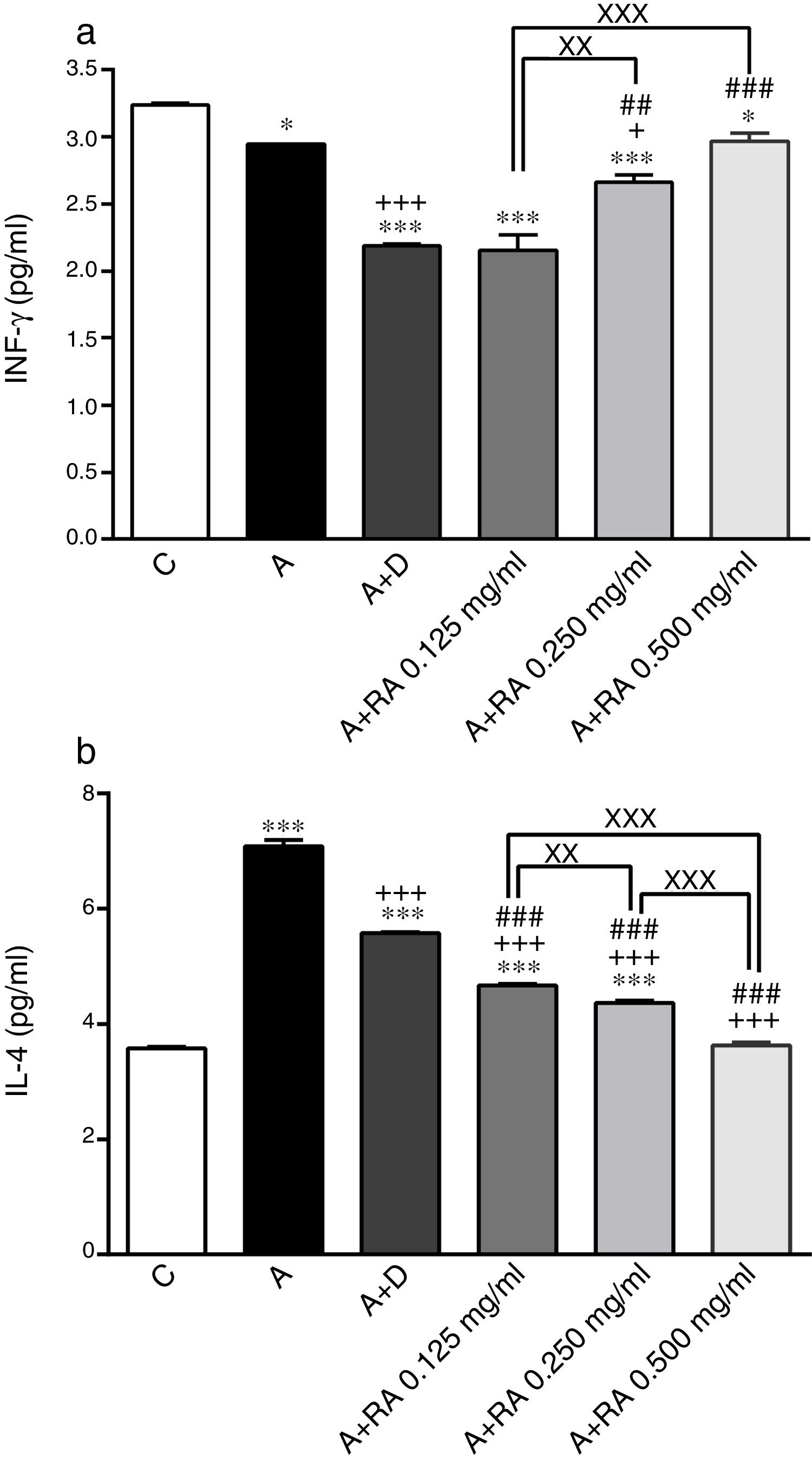
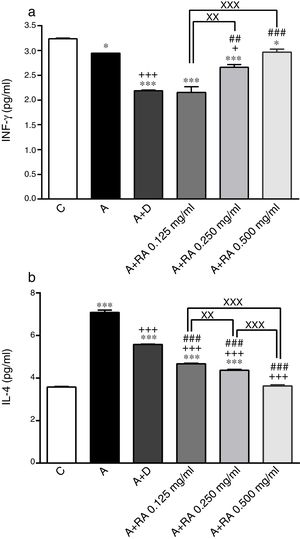
ResultsEffect of RA on immunological mediatorsThe BALF levels of IL-4 (P=0.0001) and IgE (P=0.0001) in the asthma group (group A) were significantly increased but IFN-γ (p=0.04) and IFN-γ/IL-4 (P=0.0001) ratios were decreased compared to the control group (Figs. 1 and 2). The levels of IL-4 (P=0.0001 for two lower concentrations and 0.0001 for highest concentration) and IgE (P=0.0019 for lowest concentration and P=0.0001 for two higher concentrations) in groups treated with RA (0.125, 0.250 and 0.500mg/mL) as well as the level of IFN-γ in rats treated with RA 0.125 and 0.250mg/mL (P=0.03 and P=0.0004, respectively) were significantly decreased compared to asthma group (Figs. 1 and 2).
The effect of rosmarinic acid on IFN-γ (a) and IL-4 (b) in bronchoalveolar lavage fluid (BALF) of control animals (C), asthma group (A), asthmatic groups treated with dexamethasone (A+D), (n=6 in each group) and asthmatic groups treated with rosmarinic acid (A+RA, 0.125, 0.250 and 0.500mg/ml), (RA, n=8). Data are presented as mean ± SEM. *P<0.05 and *** P<0.001 compared to group C.+P<0.05 and +++ P<0.001 compared to group A. ## P<0.01 and ### P<0.001 compared to group D. xx P<0.01 and xxx P<0.001 comparison among the three concentrations of RA. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey–Kramer's post-test.
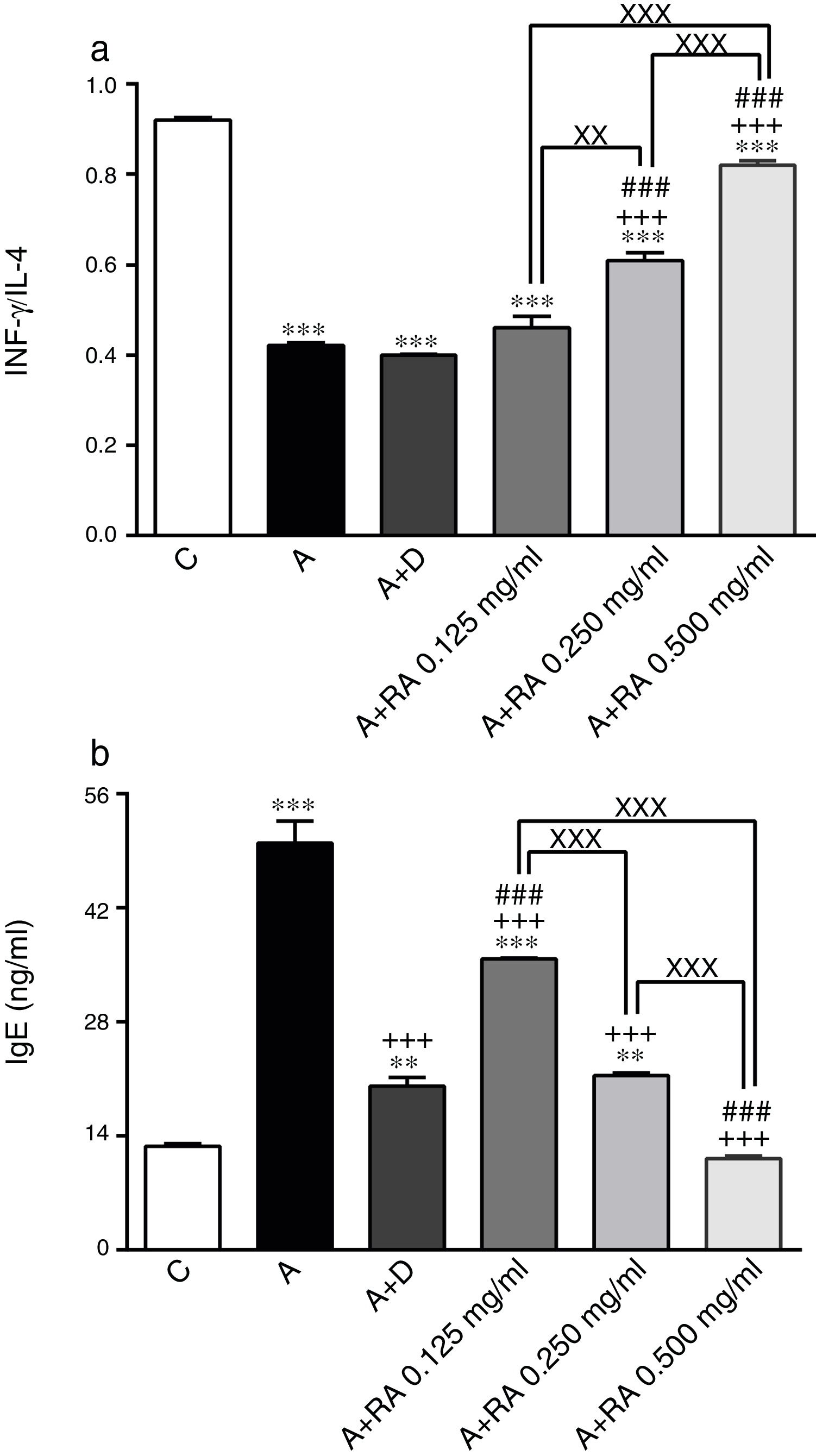
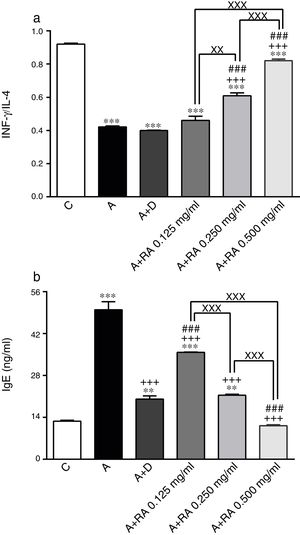
The effect of rosmarinic acid on IFN-γ/IL-4 ratio (a) and IgE (b) in bronchoalveolar lavage fluid (BALF) of control animals (C), asthma group (A), asthmatic groups treated with dexamethasone (A+D), (n=6 in each group,) and asthmatic groups treated with rosmarinic acid (A+RA, 0.125, 0.250 and 0.500mg/mL), (RA, n=8). Data are presented as mean ± SEM. ** P<0.01 and *** P<0.001 compared to group C. +++ P<0.001 compared to group A. ### P<0.001 compared to group D. xx P<0.01 and xxx P<0.001 comparison among the three concentrations of RA. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey–Kramer's post-test.
Treatment of asthmatic animals with RA 0.250 and 0.500mg/mL led to a significant increase in IFN-γ to IL-4 ratio (P=0.0004 and P=0.0001 respectively) compared to asthma group (Fig. 2). Moreover, in rats treated with RA 0.125, 0.250 and 0.500mg/mL, IFN-γ level (P=0.0001, P=0.0002, P=0.0004 and respectively), IFN-γ/IL-4 ratio (P=0.0001, P=0.0003 and P=0.0004 respectively), in rats treated with RA 0.125 and 0.250mg/mL, IL-4 level (P=0.0002 and P=0.0001) and IgE (P=0.0001 and P=0.0013) were not restored to control values and showed significant differences with those of the control group (Figs. 1 and 2).
Dexamethasone treatment caused significant reductions in IFN-γ (P=0.0001), IL-4 (P=0.0003) and IgE (P=0.0004) levels, but did not change the IFN-γ/IL-4 ratio compared to the asthma group (Figs. 1 and 2). In addition, the levels of IFN-γ (P=0.0001), IL-4 (P=0.0001) and IgE (P=0.0011) as well as the IFN-γ/IL-4 ratio (P=0.0001) in asthmatic animals treated with dexamethasone were significantly different from those of the control group (Figs. 1 and 2).
The effect of RA 0.125, 0.250 and 0.500mg/mL on the IL-4 level (P=0.0004, P=0.0003 and P=0.0001 respectively), the effect of RA 0.250 and 0.500mg/mL on the IFN-γ level (P=0.0024 and P=0.0003) and the IFN-γ/IL-4 ratio (P=0.0008 and P=0.0006), and the effect of RA 0.500mg/mL on the IgE level (P=0.0003) were significantly higher than those of dexamethasone (Figs. 1 and 2). However, the effect of RA 0.125mg/mL on the IgE level was significantly lower than that of dexamethasone (P=0.0007; Fig. 2).
The effects of RA 0.250 and 0.500mg/mL on all immunological mediators’ levels including IL-4 (P=0.0016 and P=0.0001), IFN-γ (P=0.0085 and P=0.0009), IFN-γ/IL-4 ratio (P=0.0034 and P=0.0001) and IgE (P=0.0004 and P=0.0002)) were significantly higher than the effect of its low (0.125mg/mL) concentration (Figs. 1 and 2). RA 0.500mg/mL had more marked effects on all immunological mediators’ levels including IL-4 (P=0.0001), IFN-γ/IL-4 ratio (P=0.0002) and IgE (P=0.0004) as compared to RA 0.250mg/mL except IFN-γ (Figs. 1 and 2).
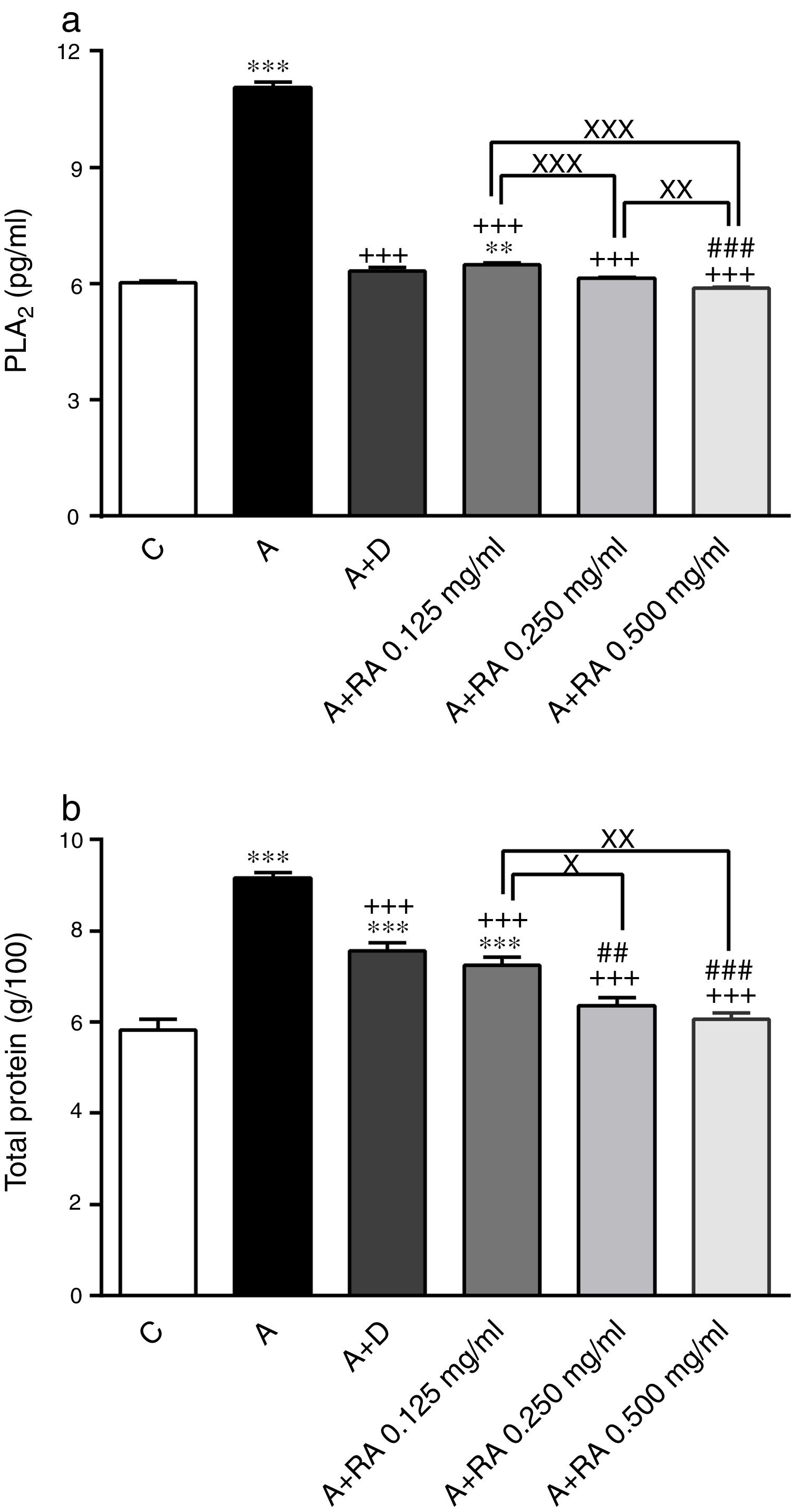
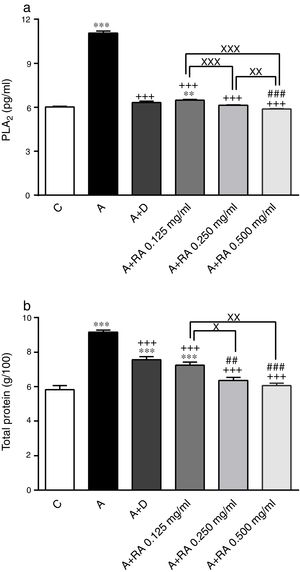
Effect of RA on inflammatory mediatorsPLA2 (P=0.0001) and TP (P=0.0001) levels in BALF of asthma group were significantly higher than those of the control group (Fig. 3).
The effect of rosmarinic acid on PLA2 (a) and TP (b) in bronchoalveolar lavage fluid (BALF) of control animals (C), asthma group (A), asthmatic groups treated with dexamethasone (A+D), (n=6 in each group) and asthmatic groups treated with rosmarinic acid (A+RA, 0.125, 0.250 and 0.500mg/mL), (RA, n=8). Data are presented as mean ± SEM. ** P<0.01 and *** P<0.001 compared to group C. +++ P<0.001 compared to group A. ## P<0.01 and ### P<0.001 compared to group D. x P<0.05, xx P<0.01 and xxx P<0.001 comparison among the three concentrations of RA. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey–Kramer's post-test.
Treatment of asthmatic animals with all concentrations of RA (RA 0.125, 0.250 and 0.500mg/mL) led to significant decreases in PLA2 (P=0.0004, P=0.0003 and P=0.0001, respectively) and TP (P=0.0003, P=0.0002 and P=0.0001, respectively) levels compared to asthma group (Fig. 3). The levels of PLA2 (P=0.0031) and TP (P=0.0004) in rats treated with a low concentration of RA were not restored to control values and were significantly different from those of the control group (Fig. 3).
Dexamethasone-treated rats showed significantly reduced levels of PLA2 (P=0.0001) and TP (P=0.0004) compared to the asthma group (Fig. 3). However, dexamethasone treatment did not restore TP levels to control values and TP levels and were significantly different from those of the control group (P=0.0003; Fig. 3).
Compared to dexamethasone, treatment with RA 0.250 and 0.500mg/mL produced more marked effects on PLA2 (P=0.0001 for both cases) and TP (P=0.0042 and P=0.0008) levels, respectively (Fig. 3).
Furthermore, RA 0.250 and 0.500mg/mL had more pronounced effects on PLA2 (P=0.0009 and P=0.0001) and TP (P=0.0138 and P=0.0022) levels, compared to RA 0.125mg/mL. In terms of PLA2 level, RA 0.500mg/mL had more marked effects compared to RA 0.250mg/mL (P=0.005; Fig. 3).
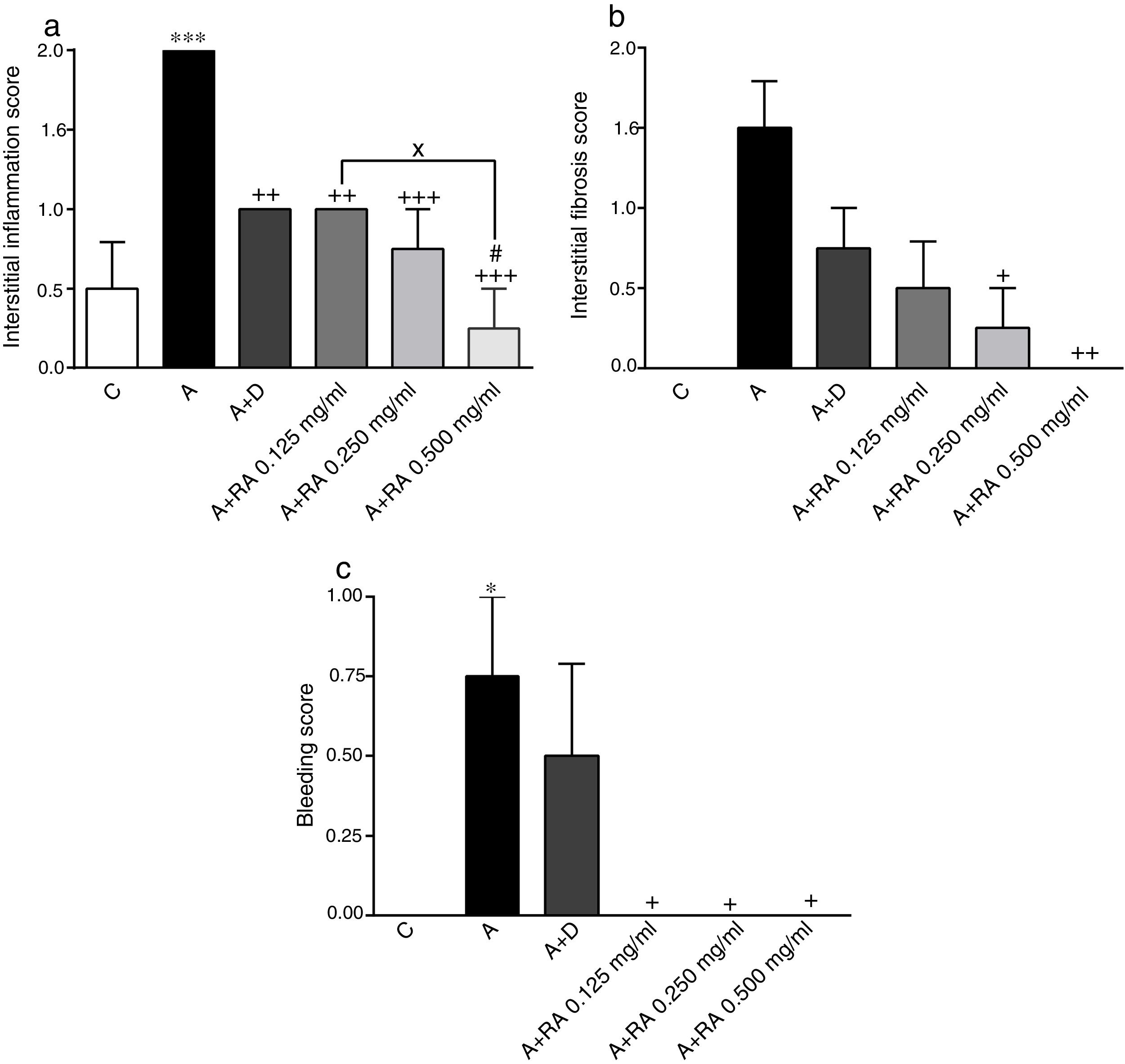
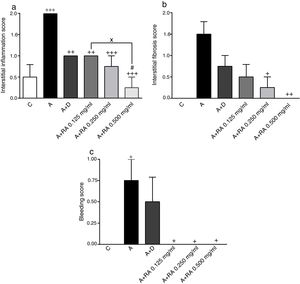
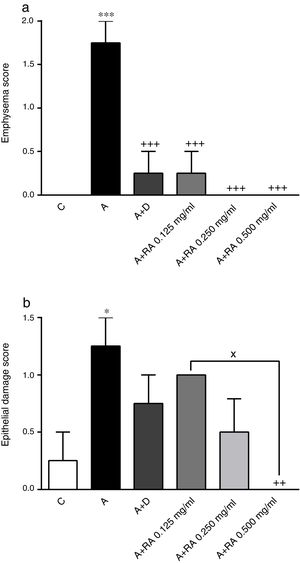
Effect of RA on lung pathological featuresThe scores of all pathological features including interstitial inflammation (P=0.0001), interstitial fibrosis (P=0.0011), bleeding (P=0.0008), emphysema (P=0.0001) and epithelial damage (P=0.03)) in the asthma group were significantly increased compared to the control group (Figs. 4 and 5).
The effect of rosmarinic acid on interstitial inflammation (a), interstitial fibrosis (b) and bleeding scores in control animals (C), asthma group (A), asthmatic groups treated with dexamethasone (A+D), (n=6 in each group) and asthmatic groups treated with rosmarinic acid (A+RA, 0.125, 0.250 and 0.500mg/mL), (RA, n=8). Data are presented as mean ± SEM. * P<0.05, ** P<0.01 and *** P<0.001 compared to group C. + P<0.05, ++ P<0.01 and+++ P<0.001 compared to group A. #p<0.05 compared to group D. x P<0.05 comparison among the three concentrations of RA. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey–Kramer's post-test.
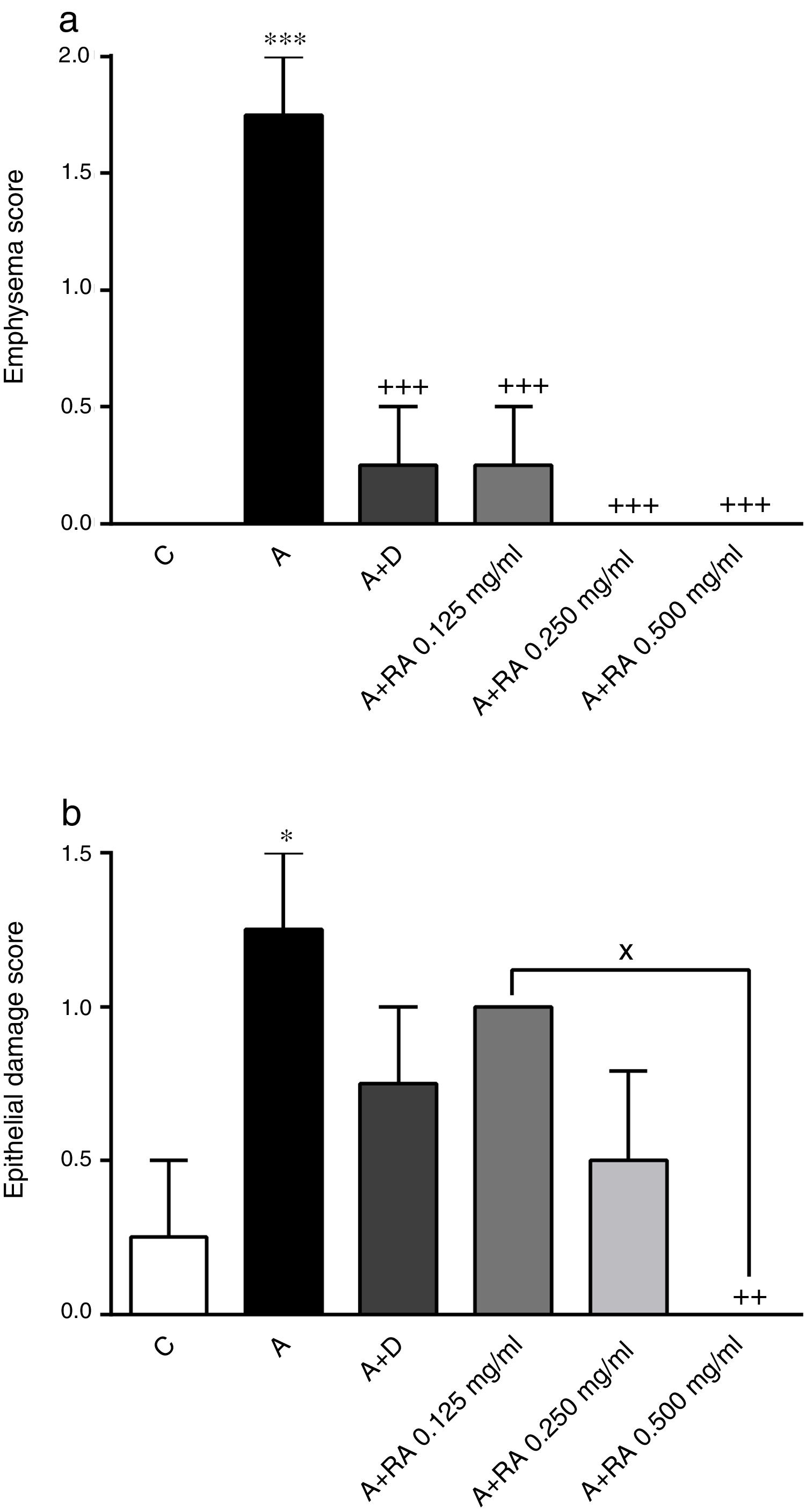
The effect of rosmarinic acid on emphysema (a) and epithelial damage scores in control animals (C), asthma group (A), asthmatic groups treated with dexamethasone (A+D), (n=6 in each group) and asthmatic groups treated with rosmarinic acid (A+RA, 0.125, 0.250 and 0.500mg/mL), (RA, n=8). Data are presented as mean ± SEM. * P<0.05 and *** P<0.001 compared to group C. ++ P<0.01 and +++ P<0.001 compared to group A. x P<0.05 comparison among the three concentrations of RA. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Tukey–Kramer's post-test.
In asthma groups treated with RA 0.125, 0.250 and 0.500mg/mL, interstitial inflammation (P=0.001, P=0.0003 and P=0.0001 respectively), bleeding (P=0.02 for all three cases) and emphysema (P=0.0004 for lower concentration and P=0.0001 for two higher concentrations), in rats treated with RA 0.250 and 0.500mg/mL, interstitial fibrosis (P=0.0170 and P=0.0043) and in rats with RA 0.500mg/mL, epithelial damage (P=0.0028) were significantly reduced compared to asthma group (Figs. 5 and 6).
Compared to the asthma group, dexamethasone-treated rats showed a significant reduction in interstitial inflammation (P=0.001) and emphysema (P=0.0004) scores, but no differences in terms of interstitial fibrosis, bleeding and epithelial damage were observed (Figs. 4 and 5).
There were no significant differences in pathological changes between control group and groups treated with dexamethasone and all concentrations of RA (Figs. 4 and 5).
Interstitial inflammation in the group treated with high concentration of RA was significantly higher than dexamethasone-treated group (P=0.0346; Fig. 4).
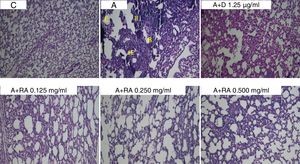
Also, RA 0.500mg/mL treatment caused more marked effects on interstitial inflammation (P=0.03) and epithelial damage (P=0.01) scores compared to RA 0.125mg/mL (Figs. 4 and 5). Figure 6 shows a specimen of lung photograph in each studied group.
DiscussionIn the present study, the effect of RA on lung inflammation in OVA-sensitized asthmatic rats, the effect of RA on BALF levels of IFN-γ, IL-4, IgE, PLA2 and TP, as well as lung pathological features were examined.
In asthmatic rats, IL-4, IgE, PLA2 and TP levels were increased but IFN-γ levels and the IFN-γ/IL-4 ratio were decreased compared to the control group confirming the induction of asthma (sensitization). In fact, the results of the present study were similar to a previous study, using a similar method of sensitization.7 Moreover, asthmatic pathological insults including interstitial inflammation, interstitial fibrosis, bleeding, epithelial damage and emphysema were observed in lung tissues of asthmatic animals. The pathological changes observed in asthmatic rats were similar to those reported by some previous studies.23,24 All these observations confirmed the sensitization of rats or induction of animal model of asthma in the present study.
In this study, treatment of asthmatic animals with RA 0.125, 0.250 and 0.500mg/mL significantly reduced IFN-γ, IL-4, IgE, PLA2 and TP levels, diminished interstitial inflammation, interstitial fibrosis, bleeding, epithelial damage and emphysema but increased IFN-γ/IL-4 ratio.
These results indicate anti-inflammatory and immunomodulatory properties of RA. Therefore, RA may have a preventive effect on asthma by the reduction of inflammation of lung and modulation of immunological mediators.
A previous study indicated that RA (5, 10 or 20mg/kg, i.p.) significantly decreases BALF levels of IL-4 and IgE in OVA-sensitized mice.18 It was reported that oral administration of RA in perilla (Perilla frutescens) extract (1.5mg/mouse/day) significantly decreases BALF level of TP in mice with Dermatophagoides farina-induced allergic inflammation.25 In OVA-sensitized mice treated with RA (20mg/kg, i.p.), IL-4, IgE and TP levels in BALF were significantly reduced.26 In mice with Blomia tropicalis-induced respiratory allergy also, administration of RA (2, 20 or 200mg/kg, i.p.) significantly reduced BLAF levels of IL-4 and IgE.27 Furthermore, decreased BLAF levels of TP in acute lung injury induced by lipopolysaccharide in mice by RA (5, 10 or 20mg/kg, i.p.) were also shown.28 In another study, RA isolated from methanolic extract of Cordia verbenacea significantly inhibited PLA2 activity in paw edema induced by Bothrops jararacussu.29
The effects of RA on pathological insults in lungs of OVA-sensitized mice showed that this compound suppresses inflammatory cell infiltration, mucus overproduction and goblet cell hyperplasia.18,26 Also, RA as a main constituent of perilla extract, ameliorated histological changes such as mucus cell accumulation and airway inflammation in a mice model of D. farina-induced allergic inflammation.25 All the above-described studies support the findings of the present study and indicate the preventive therapeutic effect of RA on asthma.
The effects of RA on all measured parameters in asthmatic rats were concentration dependent and comparable to the effect of dexamethasone. These findings also support the possible therapeutic potential of RA in term of its immunomodulatory effect and effect on pathological changes in asthma.
Over the last decades, asthma prevalence, morbidity and mortality have augmented especially in children and adverse effects are experienced following administration of conventional drugs used for its treatment.30,31 Therefore, there is a great need for development of novel anti-asthmatic agents with fewer side effects.
In this study, the effects of RA on immunological and inflammatory mediators as well as lung pathological insults were investigated in OVA-sensitized rats as an animal model of asthma for the first time. The effect of RA on tracheal responses, lung inflammatory cells and oxidant-antioxidant parameters comparable to that of dexamethasone in sensitized rats were shown previously.19 However, the effect of RA on asthmatic patients should also be examined in clinical studies. The preventive effect of RA on immunological and inflammatory mediators as well as lung pathological changes observed in this experiment together with other studies emphasize anti-inflammatory and immunomodulatory effects of RA and suggest that it as a promising therapeutic agent for the treatment of asthma. However, further experimental studies are required to demonstrate the exact underlying mechanism of action of RA in this regard. Also, clinical trials should be conducted to investigate the efficacy of RA against allergic disorders and airway diseases such as asthma.
ConclusionThis study showed that RA improves immunological and inflammatory mediator levels, and ameliorates lung pathological insults in asthmatic rats which were comparable with those of dexamethasone. Therefore, RA could be potentially regarded as a therapeutic agent to be used for the treatment of asthma.
Conflict of interestThe authors have no conflict of interest to declare.
This study was financially supported by the Vice chancellor of Research, Mashhad University of Medical Sciences and Biology Department, Ferdowsi University of Mashhad. The results of this paper are from a PhD thesis completed by Naeima Eftekhar.