INTRODUCTION
Exacerbations are the main cause of quality of life impairment in asthmatic children. Clinical and experimental evidence suggests an important role for respiratory infections in the development of asthma attacks 1-5. The first epidemiological data establishing a relationship between viral infections and asthma were obtained in the 1970s and 1980s by viral isolation and serologic techniques 6. Viral infection was identified in 31.9 % of asthma exacerbations in childhood (Pattemore et al, 1992). The three most frequently isolated viruses were rhinovirus, respiratory syncytial virus (RSV) and parainfluenza virus, which were detected in 8.8, 6.4 and 6 % of cases, respectively. The development of polymerase chain reaction (PCR) for the diagnosis of respiratory viral infections has led to studies revealing the importance of viral infections in acute exacerbations of asthma 7. An epidemiological study by Johnston et al 8 using PCR and culture detected viral upper respiratory tract infections in 85 % of childhood asthma exacerbations and in over half of adult exacerbations. Rhinoviruses accounted for 61 % of the viruses detected (coronavirus 16 %, influenza 9 %, parainfluenza 9 % and RSV 5 %). These upper respiratory tract infections are probably responsible for seasonal peaks of asthma-related hospital admissions 9,10. Human rhinovirus has been implicated as the principal virus associated with asthma episodes in school children, especially during spring and fall 1,11-13. Co-infection with other virus may be important 2,16. RSV are more important in children under two years of age 13-16. Recent attention has been directed to the role of infections with atypical bacteria such as Chlamydia pneumoniae and Mycoplasma pneumoniae, as agents capable of triggering asthma exacerbations in older children 1,3,6,13,14,17,18.
The present study evaluates the seasonal trends and the role of these pathogens in asthma exacerbations in school children from Oporto (Portugal).
METHODS
Patients
The study comprised all children aged 6 to 13 years old with asthma exacerbation, attended in the Pediatrics Emergency Department during one day per week from January 1 to December 31, 2003. Asthma diagnosis was based on the criteria of the new 2002 Global Initiative for Asthma (GINA) guidelines 19. The patients who did not meet the GINA criteria for asthma diagnosis, and patients whose parents did not give informed consent, were excluded.
Data collection and analysis
Participants were questioned in detail about common cold symptoms, previous asthma diagnosis, age at onset of asthma and rhinitis symptoms, and asthma management and control. The questionnaire was composed of 5 demographic questions and 7 health related questions. The severity of asthma and of asthma exacerbation was recorded. Nasal aspirates were obtained through nasal wash with 1 ml saline and processed by immunofluorescence assays (IFA) for RSV, adenovirus, parainfluenza and influenza virus, retrotranscription polymerase chain reaction (RT-PCR) for rhinovirus, and polymerase chain reaction (PCR) for enterovirus, Chlamydia pneumoniae and Mycoplasma pneumoniae. The diagnosis and classification of asthma, asthma severity and asthma exacerbation were based on the criteria of the new 2002 Global Initiative for Asthma (GINA) guidelines 19. The statistical analysis was performed using the SPSS statistical package (Version 11.0 for Microsoft Windows; SPSS, Chicago, IL, USA). The associations between isolated pathogens and the demographic data, severity of asthma and asthma exacerbation and current therapy were determined using the χ 2 test. A p-value < 0.05 was considered statistically significant.
RESULTS
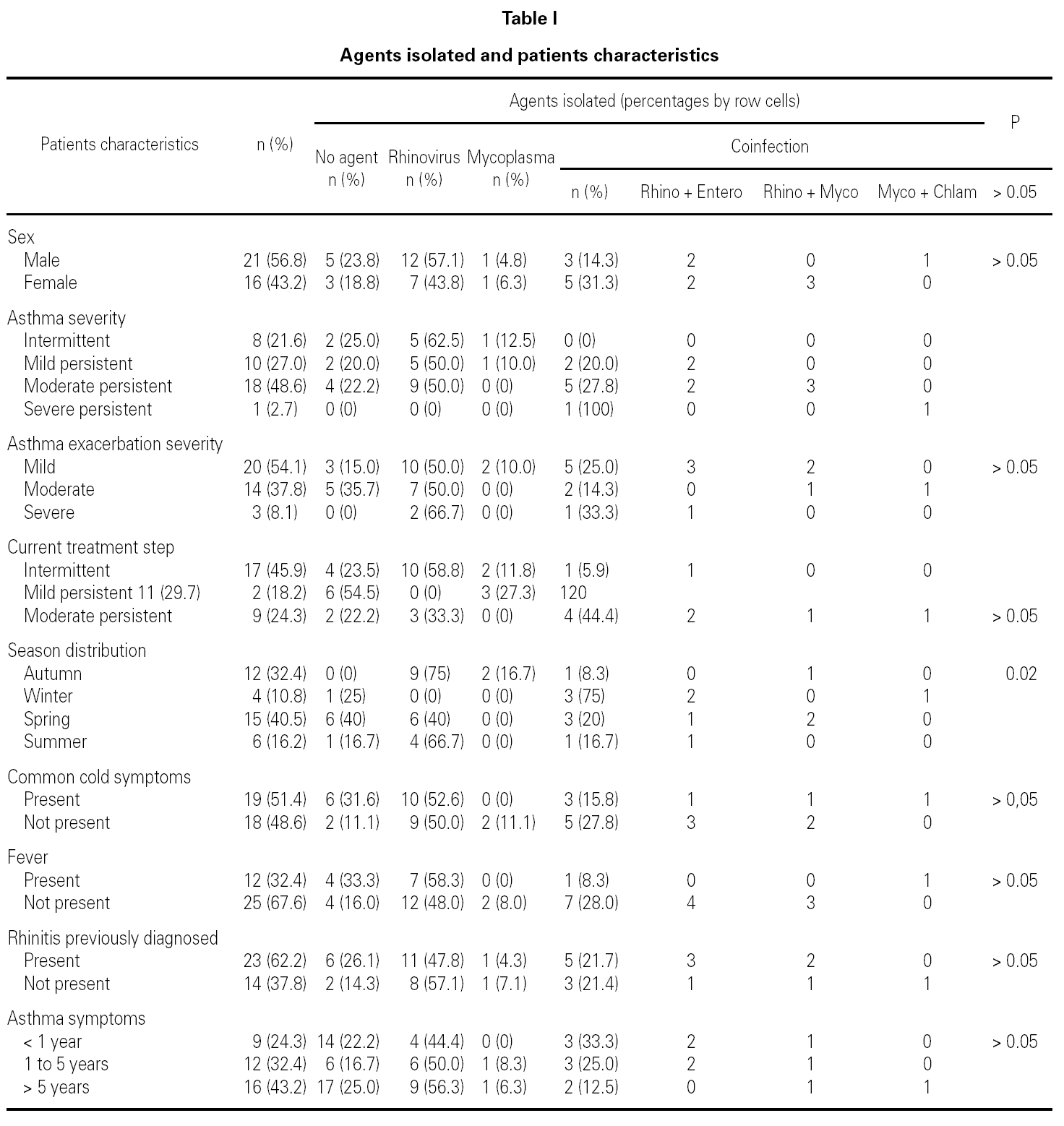
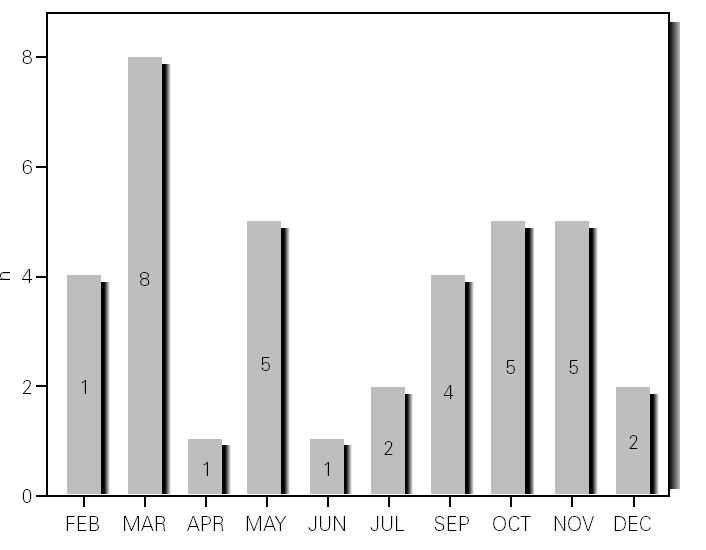
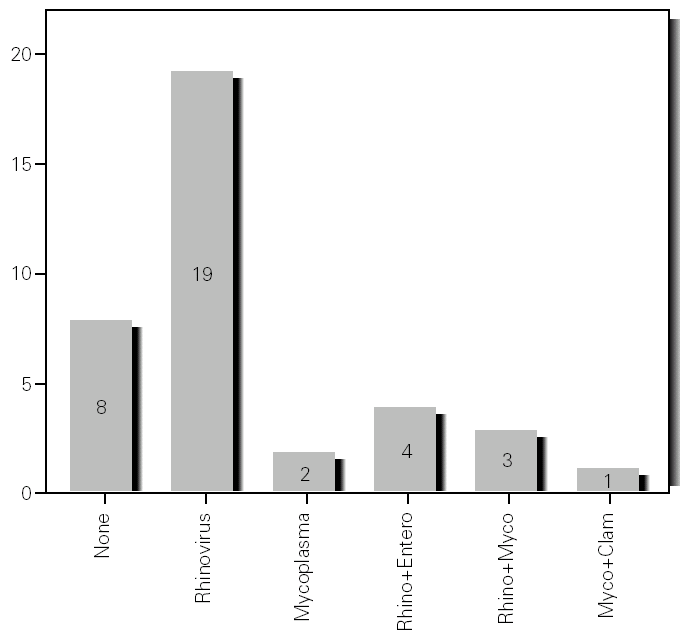
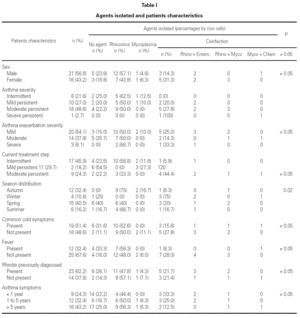
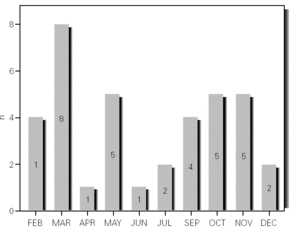
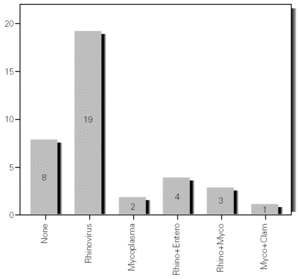
During the 12-month study period, 54 patients were screened for eligibility. Clinical reviews and agreement resulted in 17 exclusions. Data from the remaining 37 patients were analyzed. Twenty-one patients were males (56.8 %) with a mean age of 8.5 years (6 to 13). Asthma had already been diagnosed in 83.3 % of the children (in 43.2 % more than 5 years before). Fever was present in 32.4 %, and common cold symptoms in 51.4 % of the cases. Symptoms of rhinitis were recorded in 64.8 %. Asthma severity was intermittent in 21.6 %, mild-persistent in 27 %, moderate-persistent in 48.6 %, and severe-persistent in 2.7 % (table I). Asthma exacerbation was mild in 54.1 %, moderate in 37.8 % and severe in 8.1 % (table I). A quarter distribution was made for climatic reasons, since the weather in the last month of the season is more characteristic of the coming season. Two distinct peaks of asthma exacerbation were found, between September and November and between March and May. March had the highest and August and January the lowest number of cases (fig. 1). The seasonal distribution also showed two distinct peaks of asthma exacerbations: 40.5 % in spring and 32.4 % in autumn. The combination of conventional and molecular techniques detected infectious agents in 78.4 % of the cases. Rhinovirus was detected in 70.3 %, Mycoplasma pneumoniae in 16.2 %, enterovirus in 10.8 %, and Chlamydia pneumoniae in 2.7 % (fig. 2). Co-infection was identified in 21.6 % of the samples (rhinovirus + enterovirus in four patients; rhinovirus + Mycoplasma pneumoniae in two patients, and Mycoplasma pneumoniae + Chlamydia pneumoniae in one patient). There was no significant correlation between isolated agents and the month of year, though rhinoviruses were detected in the spring in 24.3 % of the cases, and in autumn in 27.0 % of the cases (p = 0.02) (table I; fig. 3). All isolated enteroviruses were associated with rhinovirus infection, and were mostly found in children with intermittent asthma (table I). Half of the cases of Mycoplasma pneumoniae isolation showed co-infection (rhinovirus in two cases and Chlamydia pneumoniae in one case). There was no significant correlation among sex, age, common cold symptoms, current therapy status, severity or exacerbation of asthma, age at onset of asthma symptoms, presence of fever or rhinitis and the isolated agents. Children with current treatment step ≥ 2 showed co-infection in a larger number of cases (35 % vs 5.9 %, p = 0.09). The only case of Chlamydia pneumoniae isolation showed co-infection with Mycoplasma pneumoniae, and corresponded to a child with severe-persistent asthma.
Figure 1.--Month distribution.
Figure 2.--Agents isolated.
Figure 3.--Agents isolated by season.
DISCUSSION
Previous studies have examined the importance of viral infections in acute exacerbations of asthma. Using conventional techniques alone, the detection rate for infections agents did not exceed 30 % (Pattemore et al, 1992). With the development and use of highly sensitive molecular techniques, it was hypothesized that the rate, as well as the range, of infectious agents associated with exacerbations of asthma, would increase 5,20. Indeed, as confirmed by Johnston et al (1995), the rate of viral detection by PCR-based assays increased to 77.3 %. In the present study, infectious agents were detected in 78.4 % of the cases. In 79.4 % of these cases a virus was detected (rhinoviruses, enteroviruses or both), while in 10.3 % of the cases a bacterium was isolated (Mycoplasma pneumoniae or Chlamydia pneumoniae), and in 10.3 % both a virus (rhinovirus) and a bacterium (Mycoplasma pneumoniae) were found (fig. 2). In addition, we noted that rhinovirus and Mycoplasma pneumoniae, found in 70.3 % and 16.2 % of the cases, respectively, were detected more frequently than other agents.
Rhinovirus was the most common agent found in the nasal samples studied (70.3 %), this being in accordance with several other studies that define this agent as the major cause of asthma exacerbations in school children 5,7,15,20,21. Co-infection with other viruses or bacteria may be important, and has been found in other studies 4,20. Co-infection was detected in 21.6 % of the cases, all of them corresponding to children with severe-persistent asthma.
A significant seasonal pattern in rhinovirus detection (27 % in autumn and 24.3 % in spring) was observed. These results support the fact that upper respiratory tract infections, especially by rhinovirus, are probably responsible for seasonal peaks of asthma-related hospital admissions, as suggested by several studies 8-10 occurring mostly in spring and fall. Eight patients that were excluded because they did not give informed consent came to the Pediatric Emergencies Department with asthma exacerbation in March and April, which explains the low number of cases included corresponding to these months.
The ability of rhinovirus to produce common cold-like symptoms in healthy individuals while producing significant alterations in lower airway physiology in asthmatic patients, would indicate that either the immunoinflammatory response to the virus is different in patients with asthma, or the consequences of this response in the lower airway is altered, or both 14. This is supported by the observation in this study that 14 patients with rhinovirus isolation did not have common cold symptoms.
Experimentally induced rhinovirus infections in patients with allergic rhinitis can alter the pattern of their lower airway response to allergen challenge, by significantly enhancing their propensity to develop late asthmatic responses 14,22. The interaction between atopia and asthma and viral infections appears to be bidirectional and dynamic in that the atopic state can influence the lower airway response to viral infections 23,24. The atopia state was not questioned in this study, but there was no correlation between the presence of previously diagnosed rhinitis and the agents isolated.
No RSV, adenoviruses, influenza viruses or parainfluenza viruses where isolated. RSV is a common cold virus and a major cause of acute asthma exacerbations, predominantly in children under two years of age 8,13,25,26. The fact that no RSV was found in nasal samples in our study can be explained by the weaker detection rate of the isolation method used: all these viruses where assessed by IFA. Another possible explanation is the lesser importance of this agent as a cause of asthma exacerbation in school children.
Since bacterial infections are known to impair mucociliary clearance and to increase mucus production in the lung, it has been proposed that certain bacterial infections may cause chronic lower airway inflammation 27. The organisms primarily implicated in this process include Chlamydia pneumoniae and Mycoplasma pneumoniae27,28. These atypical bacterial infections may precipitate asthma exacerbations, and were linked to prolonged asthmatic symptoms 1,2,7,18. In school children with wheezing, an unexpectedly high prevalence of low-grade Chlamydia pneumoniae infection also has been reported 29. Cunningham et al 29 studied 108 children (age range 9-11 years) with asthma symptoms longitudinally followed-up on (for 13 months) by means of a daily diary of respiratory symptoms and peak flow rates. Nasal aspirates were obtained when respiratory symptoms were reported. The presence of infection was investigated by PCR for Chlamydia pneumoniae, and detections were similar in the symptomatic and asymptomatic episodes (23 % vs 28 %, respectively). Children who reported multiple episodes also tended to remain PCR-positive for this agent, suggesting the presence of chronic infection. Mycoplasma pneumoniae also has been associated with chronic asthma, and infection has been documented in about 25 % of children with wheezing 7,30. In the present study we found a lower prevalence of these agents Mycoplasma pneumoniae being found in 16.4 % and Chlamydia pneumoniae in only 2.7 % of the cases. Mycoplasma pneumoniae was the second most frequently isolated agent, but Chlamydia pneumoniae did not seem particularly involved in children with acute asthma exacerbation, as found in other studies 14. There was no significant correlation to asthma severity, although the only case of Chlamydia pneumoniae detection corresponded to a co-infection with Mycoplasma pneumoniae in a child presenting severe-persistent asthma. This is an isolated case, but suggests that this agent may be associated with a more severe asthma course.
CONCLUSION
Our results confirm a high frequency of rhinovirus detection in asthmatic exacerbations in school children, and support the fact that the seasonal pattern of asthma exacerbations is due to rhinovirus infection occurring mostly in spring and fall. The findings also underscore the frequency of Mycoplasma pneumoniae detection, and emphasize the importance of this agent as a possible trigger of asthma exacerbations.
Correspondence:
M. João Silva
Rua das Mimosas 39
Milheiros
4475-079 Maia
Portugal
Phone: 00351966123421
Fax: 00351229600017
E-mail: martajoaosilva@iol.pt