Chronic rhinosinusitis (CRS) is treated with both surgical and medication options. However, long term data on patient outcomes is rare. In a real world clinical environment, our objective was to identify CRS patients, gather patient characteristics, and follow symptoms over one year.
Patients and methodsThis observational study enrolled patients with CRS. Primary clinical markers included atopy testing, serum IgE, and complete blood counts. A sinus computerized tomography (CT) scan was performed serially. Patients were enrolled into medical treatment Arm A and into surgical treatment Arm B. Symptom scores were calculated using the chronic sinusitis survey (CSS).
ResultsAtopy testing was positive in 67%. IgE levels or atopy did not correlate with CSS scores. A 23% decrease in total CSS scores was noted in Arm A at one year (P=.01). Arm B demonstrated a 38% reduction in total CSS scores at 3 months (P=.02) only. CT evidence of CRS was found in 74% of patients. However, CT scores did not change significantly over 12 months.
ConclusionsNo correlation was found between serum IgE levels or atopy versus CSS scores. CT scan scores did not change significantly over 12 months in either treatment group. A reduction of CSS scores was seen in both treatment groups; however a rebound effect was suggested in the surgical arm. Our study demonstrates the disconnection between clinical markers, radiographic evidence and response to therapy in CRS in a common clinical setting. It exemplifies the need for controlled studies with years of chronic rhinosinusitis outcome analysis.
Chronic rhinosinusitis (CRS) affects an estimated 30 million Americans in all age groups.1 Despite its prevalence and associated morbidity, CRS remains a poorly defined entity in its pathogenesis and treatment. Treatment of CRS is generally subgrouped into medical therapy and surgical therapy. Medical therapy consists of antimicrobials, nasal corticosteroids, oral corticosteroids, antihistamines, decongestants, mucolytics, mast cell stabilisers, leukotriene modifiers, nasal lavage, vaccine allergen immunotherapy and environmental modification.2 Surgical intervention has widely replaced conventional sinus surgery with functional endoscopic sinus surgery (ESS). Individual aspects of CRS medical therapy, particularly nasal corticosteroids, have been published extensively.3–5 Similarly, ESS has been shown to reduce nasal and sinus symptoms up to one year postoperatively.6 Conversely, as Khalil7 demonstrated in a large review of randomised controlled studies comparing the two arms of surgical and medical therapy, ESS does not clearly confer additional clinical benefit to medical treatment. Recent studies have showed significant benefit for the use of long term macrolide antibiotics8 or pulse dosing of oral steroids9 in chronic sinonasal disease. Overall, prior studies of CRS have compared restricted medical therapy alone or restricted medical therapy with ESS10 exemplified by Hartog et al.11 No study has undertaken an observational paradigm to prospectively follow actual CRS patients in a clinical setting of both surgery and medical therapy.
Our study attempted to reflect the real world clinical setting of CRS patient care. The goal of this study was to prospectively study CRS in patients who were followed in both an Allergy & Immunology and Otolaryngology outpatient clinic. In this observational study, our primary objective was to identify patients with the clinical diagnosis of CRS, collect baseline characteristic data of these patients, monitor radiologic evidence of disease and follow symptom scores over a one year period.
Patients and methodsOur study was an Institutional Review Board approved prospective, non-blinded, observational study, which obtained the written consent of all participants. Patients were initially evaluated in either the allergy and immunology clinic or the head and neck (Otolaryngology) clinic at the West Los Angeles Veteran's Administration (VA) Medical Center. Patients with cystic fibrosis, immunodeficiency (primary or acquired), ciliary dyskinesia or other genetic disorders affecting the nasal mucosa were excluded. Additionally, patients with known aspirin sensitivity were excluded. Patients with a history of prior ESS were also excluded. Enrolled patients required the clinical diagnosis of CRS as delineated by the guidelines from the Joint Council of Allergy, Asthma & Immunology Practice Parameters12 as well as the Rhinosinusitis Task Force of Otolaryngology- Head and Neck Surgery.13
Patients were enrolled into a medical treatment arm (Arm A) or into a surgical treatment arm (Arm B). In this VA medical center, patients were initially referred to both the Otolaryngology clinic and the allergy and immunology clinic through a primary care provider. After review of the patient's records, the patients were placed in one of two arms based upon clinical management decisions of both otolaryngologists and allergists. Patients requiring medical therapy were enrolled in Arm A. Patients who were scheduled to receive ESS were enrolled in Arm B. At the start of the study, Arm A and Arm B patients received medical therapy for CRS according to their respective primary care physician. However, the year-long follow-up of the study was conducted in the Allergy & Immunology clinic setting for both arms.
To reflect real world medical treatment of CRS, individualised medical therapy for patients in either Arm A or Arm B was not tailored to the study arm. Hence, the study did not control for the type of medication or compliance with medication used. All patients were placed on intranasal corticosteroids and antihistamines during the study except for six weeks postoperatively. Some patients were treated with sinus irrigation, leukotriene inhibitors and mast cell stabilisers intermittently. However, changes in individual medical therapy regimens and medication compliance were not explicitly recorded in this study.
Symptom scores were calculated based on severity and duration utilising the chronic sinusitis survey (CSS),14 a validated symptom survey developed to assess morbidity and quality of life. Patients were evaluated on six different occasions over a one year period and completed a CSS on each visit. Primary clinical patient markers included testing for atopy (skin prick testing or Immunocap RAST), serum IgE, and complete blood counts. Secondary markers included smoking status and serum IgG. A computerised tomography (CT) scan of the sinuses was performed initially, and after 6 months of clinical follow-up. CT scans were scored using the Modified Lund scoring system.15
Utilising both parametric and non-parametric methods, statistical analysis was conducted with a two-tailed Student t-Test to evaluate differences of means between two groups. Pearson product-moment correlation coefficient was used to evaluate for correlation between variables.
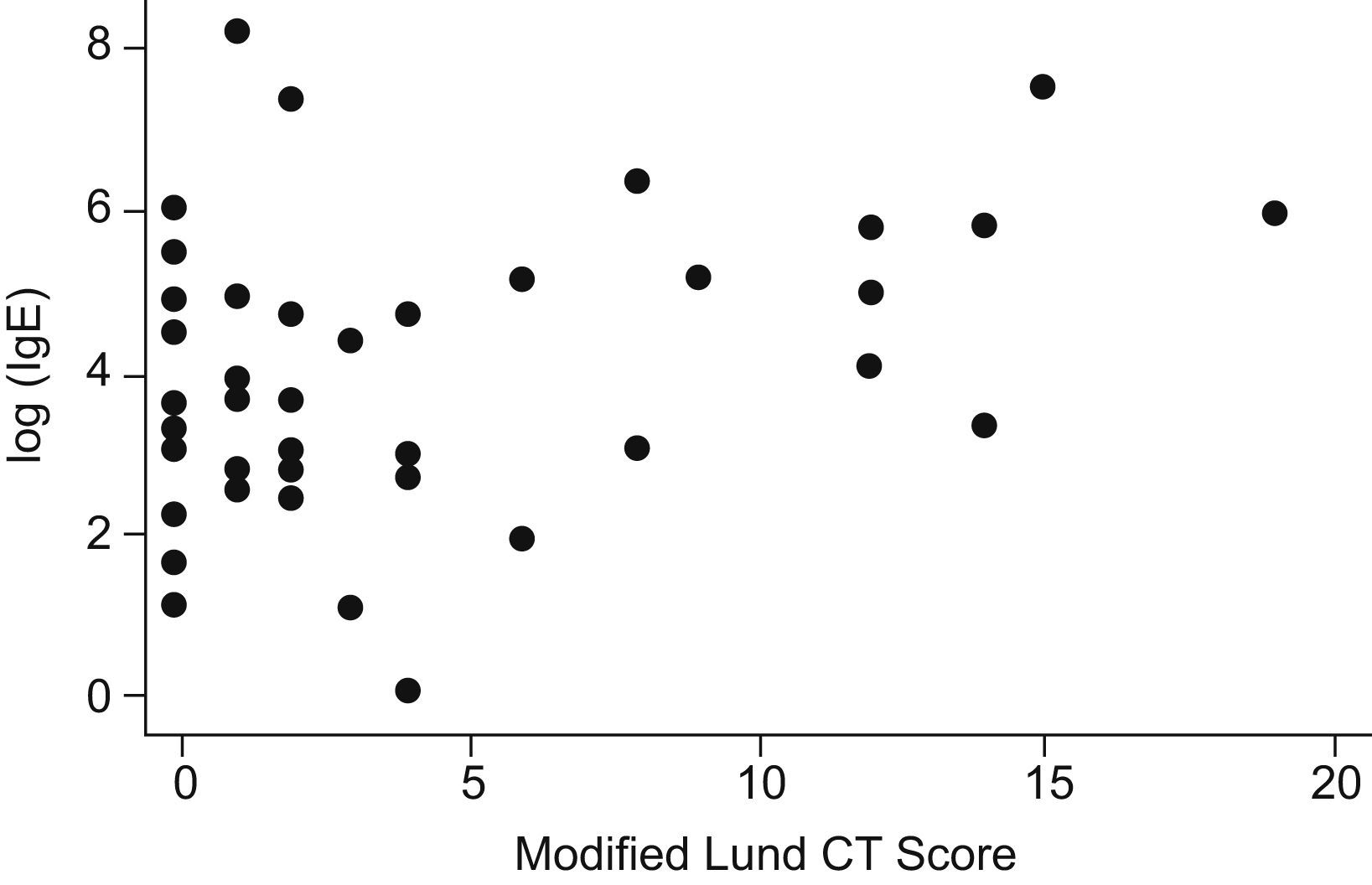
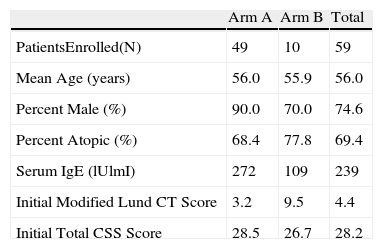
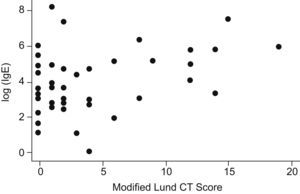
ResultsNinety-five patients and 59 patients were registered in the medical treatment arm (Arm A) and the surgical treatment arm (Arm B), respectively. In total, 49 patients completed the study in Arm A and 10 patients completed the study in Arm B (Table 1). A total of 17 patients were actively smoking during the study. The mean age of enrolled patients was 56 years. In this veterans population, males comprised 86% of the study population. RAST or skin prick testing was positive in 69% of the patients. No significant abnormalities nor correlation was noted on complete blood count or serum IgG. Seventy-four percent of patients demonstrated radiographic CT evidence of CRS. However, baseline relationships in both arms among the following variables showed no correlation. Atopy (skin prick positive) did not correlate with total CSS scores. Initial CT Lund scores did not correlate with CSS scores (r=−.03). Initial CSS score and IgE showed no correlation. However, CT Lund scores and serum IgE level showed a small positive correlation (r=.14) (Fig. 1). There was no statistical difference between smokers and non-smokers in initial total CSS score. However, in regard to the severity index of the CSS score, smokers recorded significantly higher values (P=.045).
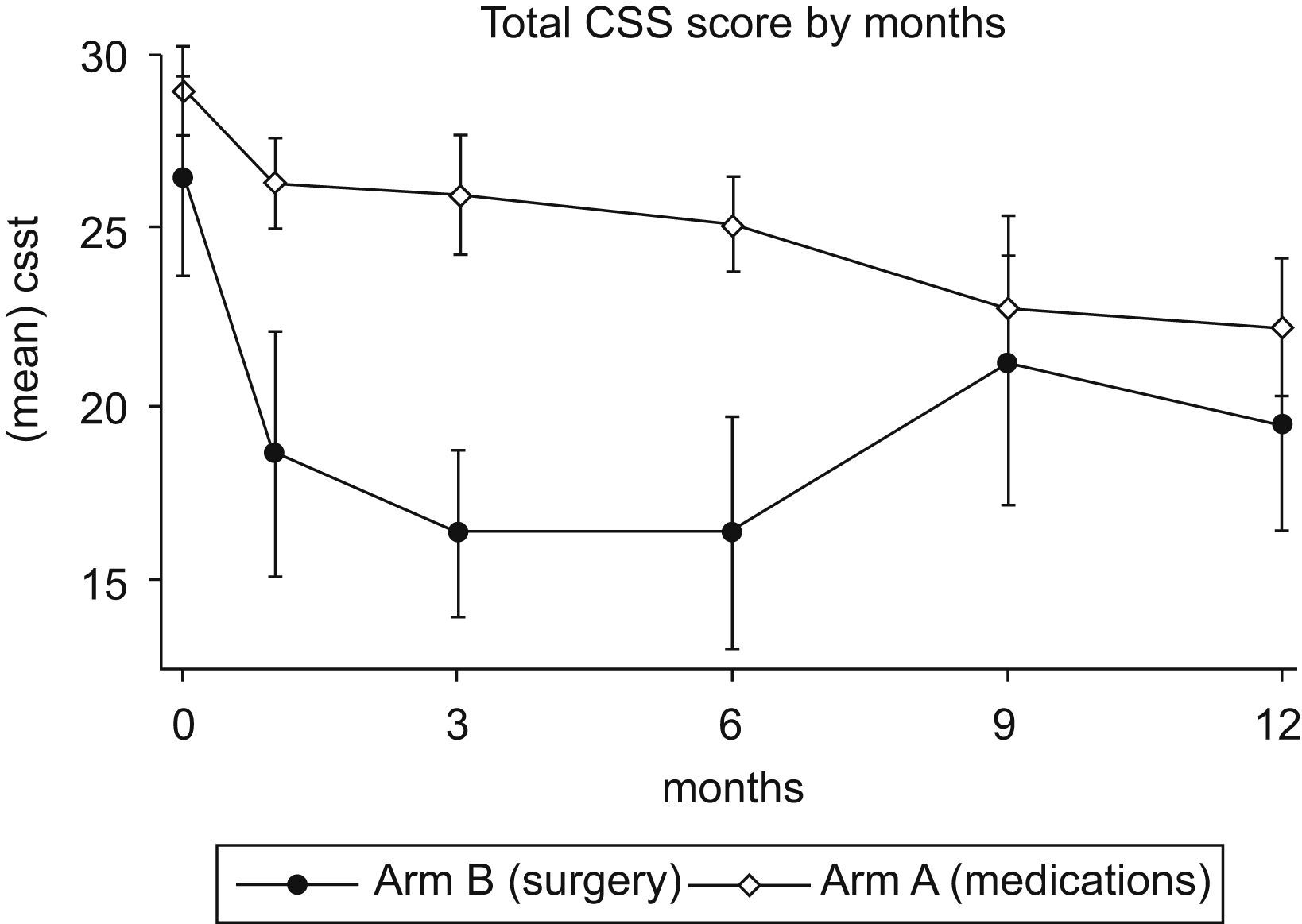
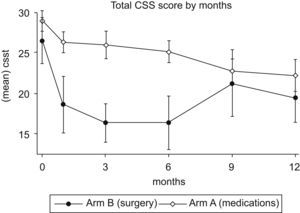
Over 12 months of follow-up, both arms showed a significant improvement in CSS scores (Fig. 2). A 23% decrease in total CSS scores was noted in Arm A at one year (P=.01). Arm B demonstrated a 38% reduction in total CSS scores at 3 months (P=.02), but at one year the reduction was no longer statistically significant (P=.07). In fact, after 3 months there was an upward trend of total CSS scores in Arm B. This trend, however, was not statistically significant.
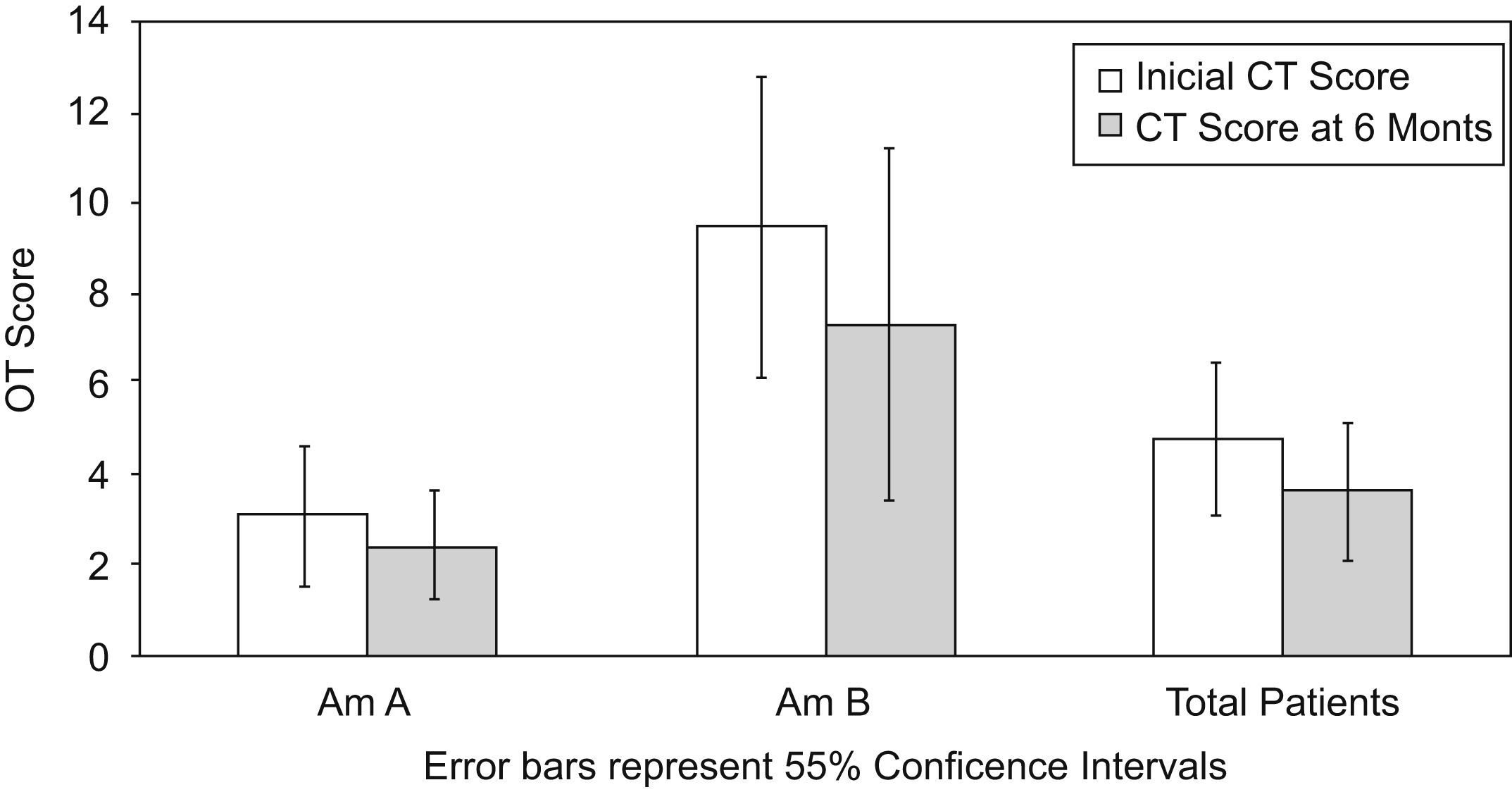
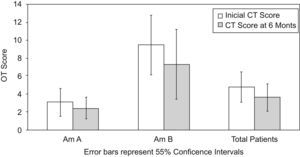
CT Lund scores demonstrated the greatest correlation of improvement in only the first 6 months of follow-up. These higher scores were exclusively in the surgical Arm B. Lund scores did not change significantly thereafter in either arm (Fig. 3). CT scores also did not vary over time by presence or absence of atopy. In both arms, Lund scores improved from baseline to follow-up. However, this improvement was not significant (P=.07).
DiscussionChronic rhinosinusitis remains a poorly characterized disorder in both otolaryngology and allergy literature. Our work attempted to follow patients in a real world setting of both subspecialties. A large attrition rate in both arms was attributed to poor patient compliance to a one year long follow-up. Despite a discrepancy in the size of the surgical treatment arm (10 patients) and the medical treatment arm (49 patients), our study was able to describe a real world comparative analysis of two common treatment approaches of CRS.
CT scan scores were higher in the surgical treatment arm. However, CT scan scores appear unreliable as up to 24% of patients with a clinical diagnosis of CRS had normal CT scan scores. Furthermore, CT scan scores did not change significantly over the first 6 months in either treatment group, nor did CT scan scores differ between atopic and non-atopic groups. Notably, a slight positive correlation was seen between IgE and CT score.
The relationship between CT score and response to treatment was poorly defined. Linear regression models suggested improvement in CT scores was significantly greater (P=.03) in the surgery group. However, this difference was no longer significant in models controlling for baseline CT scores. These scores were generally higher in the surgery group and were significantly associated with an improved change in CT score from baseline to follow-up.
In regard to patient response, a reduction of CSS scores was seen in both treatment groups. However, a rebound effect was suggested in the surgically treated arm. The maximal degree of improvement was higher in the surgery group relative to the medical management only group although this difference was not significant. This may be due to the low sample size in the surgery group which thereby affects power. The reduction in CSS scores in both medical and surgical arms is lower than previous controlled trials (Sameh et al). Our study did not control for use of specific medications or method of surgical intervention. Controlling for specific medical or surgical therapies was practically difficult in our clinical setting as two separate specialty groups with multiple physicians and surgeons prescribed individual treatment modalities to patients whose continual flux of symptoms required dynamic therapies. Instead, our study may indeed reflect more realistic responses to therapy in a setting where management is tailored to individual patients.
Correlation of subjective and objective measures was not observed. Changes in CSS scores are not related to changes in Lund scores from baseline to 12 month follow-up. Finally, in regard to atopy, our study found no correlation between serum IgE levels or skin prick testing and CSS scores. Regardless of smoking status or study arm, CSS scores were not significantly related to Lund scores. While radiologic correlates previously found a correlation between symptoms and CT noted mucosal thickening,16 the lack of correlation in our study between clinical symptom scores and radiologic CT findings reflect the conclusions of Hwang et al.17 in which the CT scan was a poor predictor of the presence of chronic sinusitis.
Our study demonstrates a disconnection between clinical markers, radiographic evidence and response to therapy in CRS. This study confirms the lack of correlation between CT scores and subjective sinusitis scores in a perioperative setting demonstrated by Bradley et al. Although utilising different sinusitis surveys, a stronger case exists to utilise CT scores independently of subjective complaints in the diagnosis and management of CRS. Despite poor utility of serum IgE and atopic markers to predict CRS response, our study also clarified that both current medical and surgical management approaches are successful in reducing CRS symptoms albeit marginally. These CRS patients continue to have significant symptoms despite either therapy modality. This study exemplifies the need for controlled studies with years of monitoring which may elucidate better methods of diagnosis and management of chronic rhinosinusitis.
We wish to thank Sabrina Lee for her efforts in organising and maintaining patient care in the Allergy & Immunology Section.