Even though there are multiple options for the treatment of asthma, there still exists a fair group of patients with difficult-to-control asthma. We describe for the first time the real-world effects of three-year omalizumab treatment on patients with difficult-to-control asthma, seen in a social security hospital in a Latin American country.
MethodsDifficult-to-control asthmatic patients from the out-patient clinic of a regional hospital were recruited to receive a three-year omalizumab course. Efficacy parameters were asthma control test (ACT) score; FEV1; daily beclomethasone maintenance dose; and unplanned visits for asthma exacerbations (emergency room (ER), hospitalisations, intensive care).
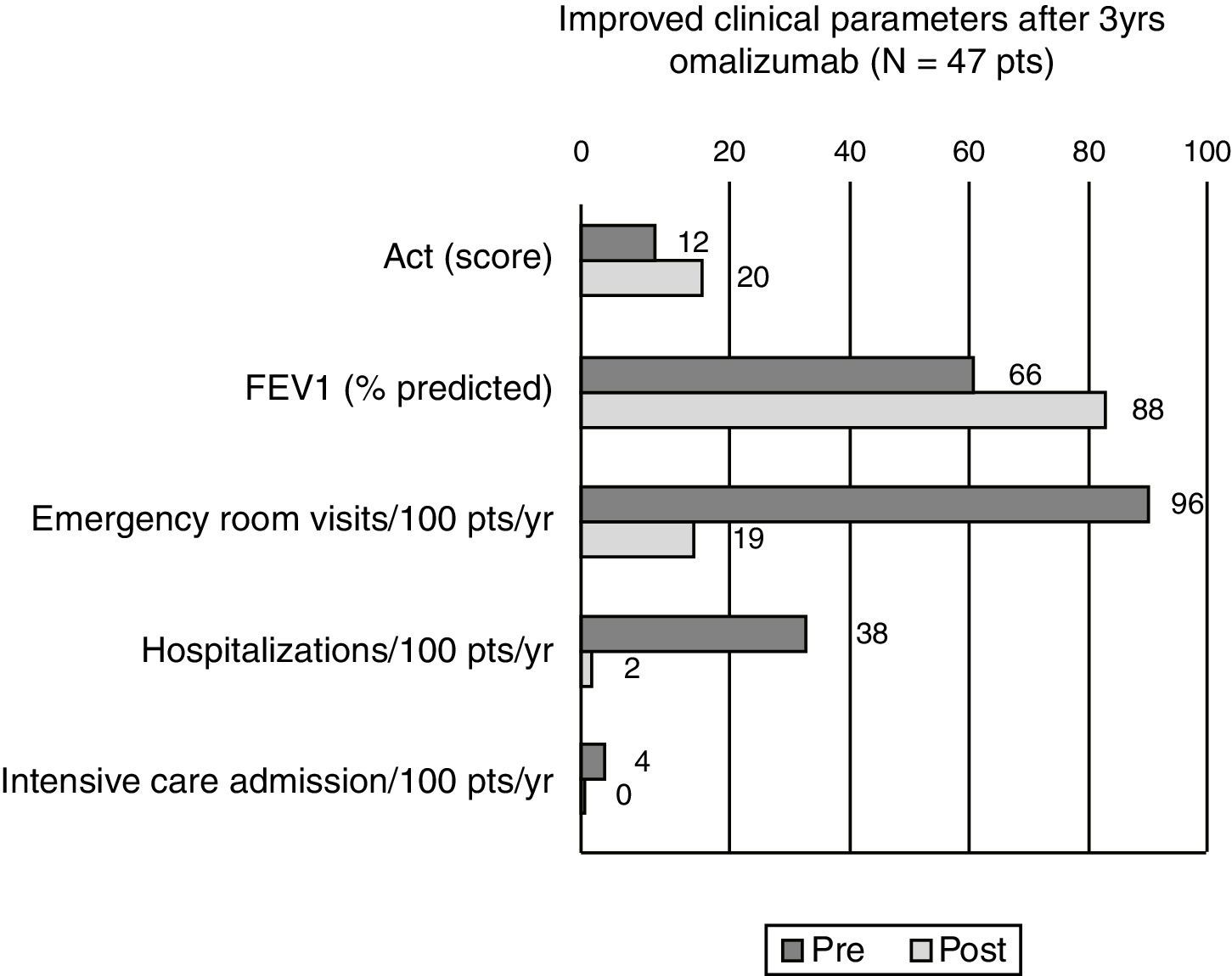
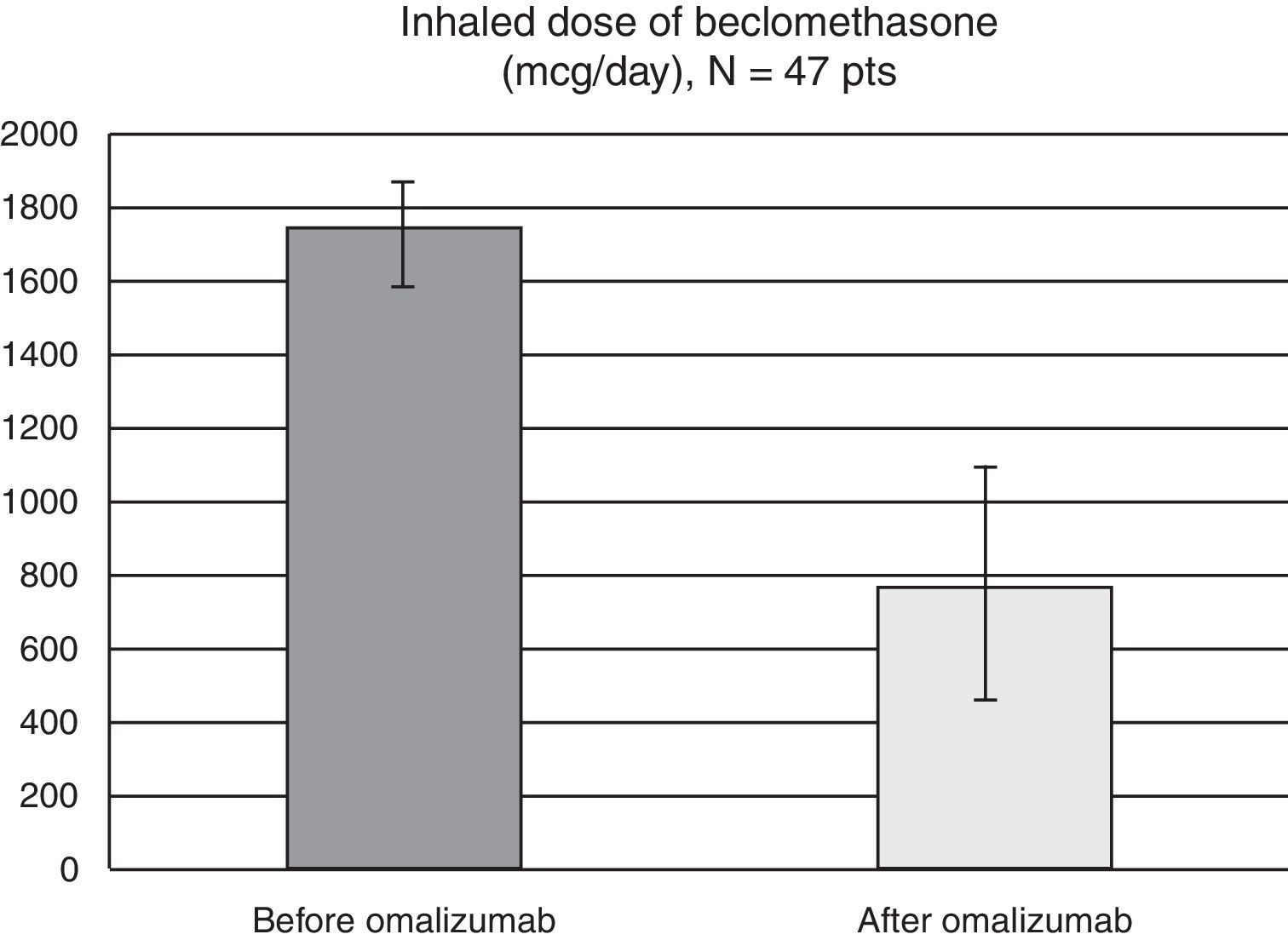
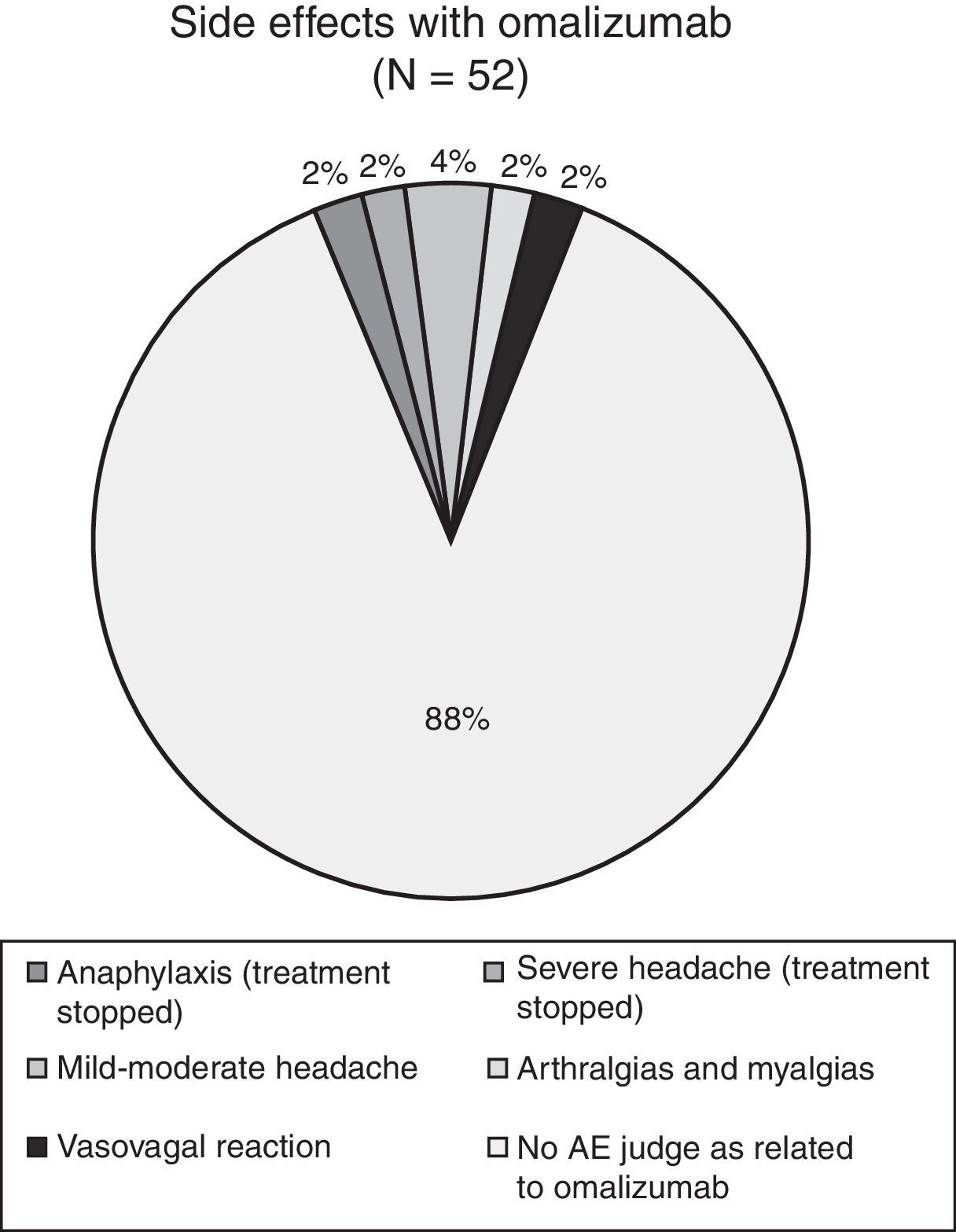
Results52 patients were recruited, 47 completed the three-year treatment (42 female, 15–67 years, mean age 43.5). Comparing efficacy parameters of the year before omalizumab with the 3rd year of omalizumab: mean ACT improved from 12.4 to 20.5, mean FEV1 from 66.3% (standard deviation (SD) 19.1%) to 88.4% (SD 16.2%) of predicted, while mean beclomethasone dose reduced from 1750 to 766mcg/day and there was a significant reduction in patients experiencing ER visits (from 95% to 19%, p<0.0001), hospitalisation (38% to 2%, p<0.0001) and intensive care (4% to 0, NS). Five patients discontinued omalizumab, two because of an adverse event (anaphylaxis, severe headache, both resolved without sequelae).
ConclusionOmalizumab improved most clinical parameters of Mexican patients with difficult-to-control asthma. Especially the rates of ER visits and hospitalisation were significantly reduced, thus reducing costs. Omalizumab was generally well tolerated.
The diagnosis of difficult-to-control asthma is considered in those patients in whom asthma is not well controlled in spite of an adequate therapeutic strategy applied by a specialist for the duration of at least six months.1 This variety and severity of asthma is complex in nature, due to the multiple phenotypes and endotypes that might lead to the persistence of the asthma symptoms, the poor response of the patient to conventional pharmacotherapy, the non-satisfactory evolution of the disease and the susceptibility of the patient to co-morbidities.2 Apart from the patients with true difficult-to-control asthma there is a considerable group of patients with seemingly difficult-to-control asthma in which the uncontrolled state of their disease is due to other factors, generally poor compliance.
Approximately 5% of all asthmatic patients develop difficult-to-control asthma, resulting in an elevated rate of complications, emergency visits, hospitalisations and even admissions to the ICU.3 That is why almost half of the total annual cost of asthma treatment corresponds to the severe asthmatic patients.4 It is important to make an effort to determine the cause of the absence of asthma control. Predisposing factors include an adverse environment, poor compliance, inadequate dosing or a level of asthma maintenance treatment unbalanced in relation to the degree of asthma severity.
Of equal importance is the detection of co-morbidities, e.g. gastro-oesophageal reflux-disease, or the differential diagnosis with other pulmonary disorders, such as chronic obstructive pulmonary disease (COPD), allergic bronchopulmonary aspergillosis, pleuropulmonar eosinophilic syndromes, Churg Strauss syndrome and occupational asthma, among others.5–7 Several treatment options have been explored to gain control in severe asthmatic patients. Inhaled and systemic corticosteroids have been the cornerstone of most treatment variants and in some cases clinically satisfactory results have been obtained as for the symptomatic and spirometric control of asthma, but the side-effects of long-term high-dose corticosteroid treatment are well-known to the medical community and close monitoring of these patients is mandatory. A small proportion of difficult-to-control asthmatic patients develop corticosteroid resistance, defined as a less than 15% improvement of the forced expiratory volume in the first second (FEV1) after a 14-day course of 40mg prednisolone. Asthma control is generally limited in these patients.5–7
Omalizumab is a humanised monoclonal antibody that binds to the constant region (Fc) of immunoglobulin E (IgE). Omalizumab blocks the binding of IgE to its high-affinity receptor Fc¿R1, situated on the surface of the mast cells (and other cells). Consequently, activation of the mast cells and liberation of its pro-inflammatory mediators is inhibited. The efficacy and safety of omalizumab in patients – six years and above – with severe asthma and difficult-to-control asthma have been documented in multiple clinical trials.8 As a consequence, omalizumab has been integrated in major clinical guidelines on the treatment of asthma, as a treatment option in the last step of therapy, in those patients not well controlled on high dose inhaled corticosteroids in combination with long-acting beta-agonists.5,9–11 Therefore, the objective of this study is to describe the results obtained after three years of treatment with omalizumab in patients with moderate to severe difficult-to-control asthma in a tertiary hospital in Mexico City.
Materials and methodsWe conducted an open prospective, observational, cohort study, describing the evolution of difficult-to-control moderate to severe asthmatic patients, recruited from the out-patients clinic of the Allergy and Immunology Department of the Regional Hospital Licenciado Adolfo López Mateos (ISSSTE), in Mexico City. Patients fulfilling the inclusion criteria were invited to a thirty-six months’ course of omalizumab. The study was implemented from January 2009 through December 2012. Patients from 12 to 75 years of age, of either sex, fulfilling the American Thoracic Society (ATS) criteria for difficult-to-control asthma6 were invited to participate and recruited into the study once an informed consent form had been signed. The following were exclusion criteria: COPD, occupational asthma, cystic fibrosis, cardiac insufficiency grade 3 or higher according to the New York Heart Association classification,12 severe pulmonary emphysema, disorders that cause laryngeal or trachea obstruction, carcinoid syndromes, hyperthydoidism, sleep apnoea and psychiatric disorders. An unacceptable dosing interval between two doses of omalizumab or a no-show of the patient for the next injection were elimination criteria.
Omalizumab (XOLAIR®) was administered subcutaneously to all patients. The omalizumab dose was calculated according to the guidelines of the manufacturer, taking into account total IgE serum levels and the patient's weight.
Depending on the administration schedule of omalizumab the patients were evaluated every 15 days or every month during the three-year surveillance. The Asthma Control Test™ (ACT) was taken at the beginning and at the end of the study.13 The ACT™ is a simple five-point questionnaire, which is self-completed by patients. Each item is scored from one (poor control) to five (good control), related to the frequency and severity of the symptoms and their impact on the patient's quality of life; the scores of each of each of the five questions are added up to give a final score with a maximum of 25. The total score of the ACT™ should be interpreted in the following way: 14 or less is considered as very poor control of asthma, 15–19 total score is un-controlled asthma, 20–24 total score reflects well controlled asthma and finally 25 points is completely controlled asthma. We compared initial ACT™ values of our patients with ACT™ scores at the end of three years of omalizumab administration to evaluate the impact of the monoclonal antibody on asthma control. Moreover, pulmonary function tests were carried out (World Spirometer Easyone 2001) and pre-bronchodilator FEV1 was recorded in percent of the predicted value, according to anthropometric characteristics, sex, race and a history of tobacco smoke exposure.
Before the study started and during the surveillance period unscheduled visits to the clinic because of asthma exacerbations were recorded. These unscheduled visits were classified according to their nature (emergency department, hospitalisation, intensive care unit (ICU)) and expressed as unscheduled visits per year. Additionally, the start dose and the final dose of beclomethasone were documented, as well as the intake of any systemic gluco-corticosteroids. No detailed record was kept on other asthma medication taken, so only a general picture can be given on this issue. A log of adverse events possibly or probably related to the administration of omalizumab was carefully updated at each clinic visit.
All included patients continued normally with their regular asthma maintenance treatment in a step-wise approach as indicated by the 2010 Global INitiative on Asthma (GINA) guidelines.5 All records were downloaded into an electronic database and we used the 19th version of the SPSS program for statistical analysis.
ResultsFifty-six patients were pre-selected as possible candidates for our study, but four patients were excluded from the trial (3 COPD, 1 sleep apnoea), leaving 52 patients who started omalizumab administration (42 females, 15–67 years, mean age 43.5 years).
The mean omalizumab dose was 279.2mg monthly. Five patients discontinued the study because of the following reasons: pulmonary tuberculosis, mild anaphylaxis, severe headache, poor compliance with the administration of the study medication and weight loss of more than 20kg. A thorough clinical history showed that the weight loss of the patient had already started before the administration of omalizumab; the patient was referred to the department of internal medicine for further investigation. The event was judged as not related to the administration of omalizumab by the investigator.
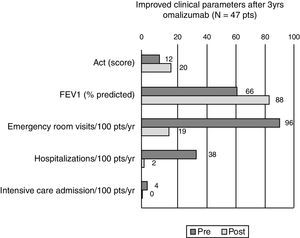
Forty-seven patients finished the three year surveillance period. The results of the per protocol analysis of the ACT™ showed a mean score of 12.4 points before the first administration of omalizumab (=very poorly controlled asthma). At the end of the three years of omalizumab administration the mean group score was 20.47 points (=controlled asthma). At patient level, the mean personal ACT™ improvement was 8.06 points (median 8, SD 3.57). Thirty-five patients (74.4%) reached the 20–25 interval of well-controlled asthma, twelve patients maintained a score between 15 and 19, thus in the range of uncontrolled asthma (Fig. 1).
Before the administration of omalizumab, at study-start, pulmonary function testing showed a general group mean FEV1 of 66.3% of the predicted values (SD 19.1%). After the three years of omalizumab the group mean FEV1 of the per protocol population had risen to 88.4% of predicted values (SD 16.2%) (Fig. 1). For the intention-to-treat analysis the mean FEV1 values changed from 66.7% pre-omalizumab to 86.1% after 36 months of omalizumab.
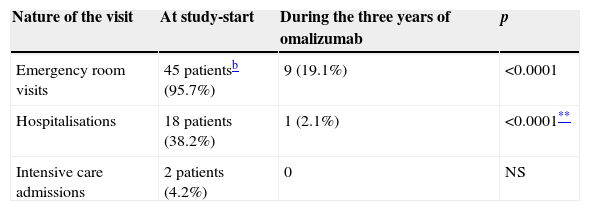
At study-start 45 patients (95.7%) had had at least one emergency visit over the past year (mean 1.94 visits/year, SD 1.3), 18 patients (38.2%), reported at least one hospitalisation due to an asthma exacerbation the year before study-start and two patients (4.2%) had been admitted to the intensive care unit (ICU) because of severe, near fatal asthma exacerbations (Table 1).
Mean number of yearlya unscheduled clinic visits for asthma exacerbations, before and at the end of three years omalizumab (N=47 pts).
| Nature of the visit | At study-start | During the three years of omalizumab | p |
|---|---|---|---|
| Emergency room visits | 45 patientsb (95.7%) | 9 (19.1%) | <0.0001 |
| Hospitalisations | 18 patients (38.2%) | 1 (2.1%) | <0.0001** |
| Intensive care admissions | 2 patients (4.2%) | 0 | NS |
NS: not significant.
After three years of omalizumab administration a clear reduction in all types of unscheduled visits was seen. During the three years of the trial, only nine (19.1%) of the 47 patients that concluded the surveillance period had an emergency room visit because of an asthma attack, only one patient (2.1%) was hospitalised and there was not one ICU admission (Fig. 1).
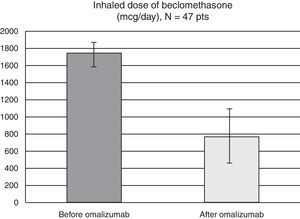
As for the inhaled corticosteroid dosing, all patients used high-dose therapy before starting omalizumab (mean 1750mcg/day, SD 345mcg). At the end of our three-year study, five patients (10.7%) were completely waned off of their inhaled corticosteroids, while the mean beclomethasone dose for the remaining 42 patients was of 765mcg/day (SD 654mcg) (Fig. 2).
The year before being put on omalizumab all patients had received continuous- or intermittently oral corticosteroids (OCS, deflazacort ranging from 24 to 60mg daily, prednisone 15 to 75mg daily and some received intravenous methylprednisolone 125 to 250mg during exacerbations. At the end of the trial, no patient was receiving daily OCS anymore; some did however still need short-time pulses for exacerbations. At study start almost all patients used LABA, specifically salmeterol 25/50mcg, MDI, at doses ranging from 50 up until 100mcg daily. However, the use of this treatment was intermittent, as this drug was often not available to our patients. In the majority of the subjects this dose was reduced or even suspended in the course of the trial, in line with the ICS dose reduction. At the end of the trial, there were still five patients receiving 25/50mcg once daily. As for Montelukast: almost all patients have been on Montelukast 10mg ante nocte throughout the study, as this is standard treatment in our hospital. A minority of patients received theophylline at the start of the study and some continued it during the trial.
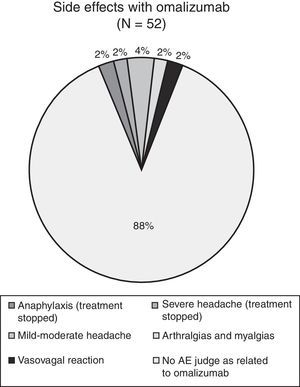
During the three years of the study four adverse events were documented (see Fig. 3): one case of osteo-articular pain, two cases of mild headache and one case of a vasovagal syncope. All four cases received specific treatment and all patients concluded the study without interrupting the administration of omalizumab. In conclusion, we registered four adverse events over the three-year period of administration of omalizumab in the 49 patients; none of the events were considered life-threatening and none of these four events led to further drop-outs.
DiscussionThe present study shows that in difficult-to-control, asthmatic patients in a specialised hospital in Mexico City, three years of omalizumab treatment resulted in an important improvement in asthma control in most patients, as well as an improved lung function and a significant reduction in unscheduled visits to the emergency room, hospitalisations and admissions to ICU due to asthma exacerbations. At the end of the study the patients showed notable clinical improvement compared to the pre-study condition of their asthma as we demonstrated with the clear rise in ACT score. At study-start all patients were classified as poorly and very poorly controlled, while at the end of three years of omalizumab two thirds had reached the level of well-controlled asthma, as expressed by the group mean ACT score of 20.47 points.
Lung function testing is one of the prime objective measurements for follow-up of asthmatic patients, especially those patients with difficult control. The three years’ treatment with omalizumab significantly improved the mean FEV1 from 66.3% to 88.4% of predicted. As such the results of the ACT and of lung-function testing are in the same line.
Finally, the most important parameter of asthma control is the rate of emergency visits and hospital/ICU admissions because of asthma exacerbations. Before study-start practically all patients reported several unscheduled visits, because of their asthma per year and even two patients had a history of ICU admission because of a near-fatal asthma attack. These numbers improved impressively with the anti-IgE antibody administration, reducing unscheduled emergency room visits to below ten percent and only one hospital admission over the last year of treatment. The impact of such a reduction is not to be under-estimated as it does not only significantly improve the patient's quality of life, but it also reduces work-loss and it reduces – in a much greater extend – direct and in-direct health care costs of asthma.
A post-marketing surveillance trial of 280 German asthmatic patients14 showed similar results to our surveillance trial: after six month omalizumab there was a reduction in unscheduled health care contacts and hospitalisations of around 80%. Twelve months omalizumab in 12–20-year-old asthmatics reduced the need for systemic corticosteroids almost to zero,15 just as in our population. Compared to these and other data published, concerning the impact of omalizumab on emergency visits and hospitalisations in real-life situations16 several factors might influence the even better results we found: the above mentioned post-marketing surveillance trials report on the effect of omalizumab administration over a shorter period of time. Post-marketing results on three years are scarce. Another factor that might play a role is the fact that our hospital is a reference hospital for patients of the social security system. It might be possible that for some patients optimal asthma management was only installed after they were referred to our clinic, months before trial recruitment, thus improving their asthma control even more than it would have improved with only omalizumab.
Over the past years asthma still remains a frequent cause of hospitalisation. Demographic and health statistics show it continuous to cause remarkable morbidity among the population of both high-level and low-level income countries.17,18
With the venue of the era of biologics for the control of diseases, these new treatment options have been shown to be effective in controlling severe forms of several disorders, uncontrolled until that moment with even the best practice. Omalizumab is a monoclonal antibody that has nowadays a well-accepted place in the management of severe asthma, as numerous clinical trials have shown its efficacy in severe cases of asthmatic airway inflammation in difficult-to-control patients. At present many guidelines have given omalizumab a place in the last step of the escalated treatment recommendations.5,10,11
Investigators had been looking for alternative treatment options that might reduce the adverse effects of prolonged high-dose corticosteroid use or, even worse, of frequent systemic gluco-corticosteroid (GCS) bursts. As such, the GCS reducing capacity of several immunosuppressive drugs was explored, e.g. chloroquin, dapsone, azathioprine, salts of gold, cyclosporine, methotrexate, to mention some. However, the clinical trials with these agents, used as GCS saver medication in severe asthma, have not been able to show consistent results. Based on the results of Cochrane meta-analysis these agents have received the level of evidence B and C, because of some contradictory outcomes. Although their use has shown some benefit in severe asthmatic patients, frequently observed safety issues and other potentially harmful effects limit their usefulness in clinical practice. As a consequence their indication in severe, difficult-to-control asthmatic patients is still a matter of debate and much room is left to the judgement of the treating physician as for the decision to prescribe or refrain from prescription in this small sub-group of asthmatics.19–24 Intravenous gamma globulin offers some benefit to GCS-dependent asthmatic patients, but here again studies show contradictory results. Additionally, the adverse events that have been described with IVIG cannot be neglected (e.g. fever, aseptic meningitis, urticaria).24
Over the past years several biological treatments have been developed, with monoclonal antibodies directed against various pro-inflammatory molecules. Tumour necrosis factor alpha (TNF-alpha) is one of the molecules involved in the pathophysiology of severe asthma.25 Trials have been designed to test anti-TNF-alpha antibodies, such as the monoclonal chimeric antibody infliximab that binds TNF-alpha and inactivates its inflammatory capacity. Preliminary results have shown an improvement in the quality of life of severe asthmatic patients, a mild improvement in lung function and a reduction in the hospitalisation rate. However, not all trails have shown positive results, some of them recruited a very limited number of patients and the reported side-effects (tuberculosis, infections, and others), have made the acceptance of infliximab as regular treatment for severe asthma poor.26
Following the same line of thinking industry has sought other monoclonal antibodies to block different molecules of inflammatory pathways in asthma. Some examples are mepolizumab, that blocks interleukin 5; lumiliximab, an anti-CD23 antibody, pascolizumab, a monoclonal antibody that interacts with interleukin 4; lebrikizumab, an anti-IL-13 antibody and inhibitor of periostin. Thus far, results have been promising, but these molecules are still in the experimental phase and consequently they have no official place in the asthma treatment yet.26–28
As such omalizumab is to date the sole treatment accepted in official guidelines in the last step of asthma treatment as corticosteroid safer, without being an immunosuppressant. In our study the mean dose of inhaled corticosteroid was significantly reduced. Those patients who completed the three years of omalizumab administration diminished the inhaled beclomethasone dose from the high to the low-middle range, without losing control of their asthma. 10.5% were even able to withdraw inhaled corticosteroids completely. As such we showed that omalizumab has an important corticoid sparing effect, reducing concomitantly the possible side-effects of prolonged ICS use.
Another prime concern in our cohort study was the safety of the administered biological substance, omalizumab; we observed our patients closely for possible side-effects. We reported one case of anaphylaxis after the second administration, a serious adverse event that has been documented in rare cases after the first several administrations of this medication.29 As more than one organ system was involved in the symptoms of our patient after contact with a substance that might cause anaphylaxis, according to international guidelines we had to catalogue the reaction as anaphylaxis,30 even though the symptoms were not of severe intensity. For safety reasons it was decided to exclude the patient from further omalizumab treatment. A recent review on this subject concluded that the data document low risk of anaphylaxis with omalizumab use. Moreover, the authors concluded that additional research is needed to elucidate the mechanism by which omalizumab causes anaphylaxis,31 as at first sight it seems a paradox that an anti-IgE antibody can induce massive mastcell degranulation.26
In general, over the three years of omalizumab treatment there were few adverse events and they were generally mild in intensity, including headache, myalgia, and arthralgia as the most frequent ones. In only one of these patients omalizumab was discontinued (severe headache).
In this respect, our real-world data are in agreement with those previously reported in a randomised, placebo controlled clinical trial with omalizumab (the INNOVATE study), in which omalizumab was administered to 419 severe asthmatic patients for 28 weeks.27 At study-start these patients were also on high dose ICS, mostly combined with long acting beta-agonists and courses of oral corticosteroids and they had FEV1 values ranging from 40 to 80% of predicted. Favourable and significant efficacy data obtained with this group of patients were similar to those reported in our cohort.
Another post-marketing study with omalizumab was conducted in Israel. However, the 47 patients included in that study were notably older than our patients. Although significant improvement was also shown in that cohort, the results were less impressive than ours.32
In conclusion, after three years of omalizumab treatment a significant reduction in the asthma severity (ACT) could be documented in patients with difficult-to-control asthma, combined with an improved lung function, a reduction in the maintenance dose of ICS. However, our prime finding is the significant reduction in the frequency of asthma exacerbations and emergency room visits and hospitalisations caused by these asthma exacerbations. This finally translates into a positive pharmaco-economic balance, even with this high-cost treatment.
Finally, it has not yet been established clearly for how long omalizumab treatment should be continued to change a difficult-to-control asthmatic patient into a well-controlled patient and to avoid relapses after discontinuation.33 Long-term studies shall have to be conducted to clarify this issue.
Ethical disclosuresProtection of human subjectsThe authors declare that no experiments were performed on humans or animals for this investigation, as it is a post-marketing trial.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
Investigators did not receive any specific funding for the study.