Isoniazid is an essential drug in the treatment and prevention of tuberculosis. It is metabolised in the liver by an acetylation process to form major and minor metabolites. The use of isoniazid may lead to hypersensitivity reactions such as fever, rash, eosinophilia, haemolytic anaemia, angiitis, and hepatitis; which may occur singly or in combination.1 The most common skin lesions are morbiliform, maculopapular or urticarial rash.2 Some rapid desensitisation protocols for isoniazid have been reported.2–4 The treatment with adalimumab, an anti-TNF monoclonal antibody, is associated with the reactivation of tuberculosis and requires a prophylactic treatment with isoniazid for nine months.5
We report the case of a 56-year-old woman with no previous history of atopic disease or other antibiotic hypersensitivity. She has been followed for rheumatoid arthritis for six years and has been put on meloxicam and sulphasalazine treatment for six years and leflunomide therapy for four years. Three months ago, she started to receive adalimumab injections twice a month. Due to this immunomodulatory treatment, she was advised to receive one isoniazid tablet of 300mg daily, a drug she had never previously taken, for tuberculosis chemoprophylaxis. The patient noticed generalised itching and erythema on her arms and legs almost 12h after the first dose; however, ignoring the symptoms, she continued the isoniazid therapy. By the time, the skin lesions developed to the typical urticarial lesions and a mild oedema in the eyelids occurred. Thus, the patient ceased taking izoniazid tablets after four days and, on presentation at the next day, physical examination revealed urticarial and papular lesions on both thighs and excoriation scars on the arms. There were no symptoms and signs concerning other body systems. The vital signs and basic laboratory findings were normal, including the liver function tests. The patient was prescribed an oral antihistamine for a week and the lesions disappeared without trace in three days. No biopsy was taken from the lesions. The patient denied having experienced any previous urticarial skin lesions.
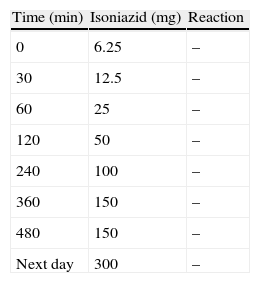
The patient was hospitalised six weeks after the reaction for drug tests and a possible intervention for desensitisation. The informed consent of the patient was obtained. The skin tests for isoniazid were negative (prick tests with 0.1mg/ml and 1mg/ml, and intradermal tests with 0.03ml of 0.1mg/ml and 1mg/ml of isoniazid). Although the skin tests did not support an IgE-mediated hypersensitivity reaction, desensitisation was considered in the light of clinical manifestation. A rapid oral desensitisation was performed the next day, following the protocol displayed in the Table 1. No reaction was observed during and after the procedure and the patient was discharged from hospital on a regimen of 300mg isoniazid daily. Previous reports recommended the use of the injectable form of isoniazid for skin tests and the use of the elixir form for the initial small doses (<50mg) of desensitisation.2 In our country, the only available forms of isoniazid are 100mg and 300mg pills. Therefore, a suspension was obtained by diluting the pill in distilled water and this was used for both the skin tests and in the desensitisation protocol.
Like most of the previous reports in the literature, the skin tests were negative in our case. This suggests a non-IgE mediated immunologic reaction to isoniazid or that the reaction was caused by a metabolite of isoniazid.2–4 Rapid desensitisation, a method classically used for type I IgE-dependent hypersensitivity, is also applicable for the cases where the presence of drug-specific IgE was not demonstrated.6 A recent consensus statement on rapid desensitisation underlined that most of the patients who had experienced hypersensitivity reactions within 24h after the last dose of drug reached tolerance with this procedure.7 Traditional desensitisation protocols for isoniazid took longer durations; therefore, they could pose a risk for resistance development. The patient has just concluded the fourth month of the prophylactic isoniazid therapy and, thanks to rapid desensitisation, the patient was able to use the drug without further hypersensitivity reaction and with less risk for resistance development. This case showed that diluted oral pills of isoniazid could be used for desensitisation when other forms were unavailable. To our knowledge, this patient is the first reported case of rapid oral desensitisation to isoniazid used for chemoprophylaxis.
Conflict of interestNone of the authors declared any conflict of interest.
FundingNo funding was received.