Both breastfeeding and the moment at which introduction to solid food occurs have been associated with food allergy.
ObjectiveTo evaluate whether prolonged breastfeeding and the delayed introduction of whole cow's milk into an infant's diet are factors that can be associated with egg sensitization.
MethodsThis was a hospital-based case–control study, matched by age and sex: each study group comprised 97 atopic children. Additionally, logistic regression was used to identify the factors associated with egg protein sensitization.
ResultsThe most common type of allergic disease among both groups was allergic rhinitis. After adjusting for possible confounding variables, a delayed introduction to whole cow's milk decreased the odds of egg protein sensitization; OR=0.16 (95% CI: 0.07–0.36, p<0.0001). Notably, breastfeeding during the first six months of life, regardless of whether it was the only milk an infant drank, increased the risk for sensitization to chicken eggs; OR=5.54 (95% CI: 2.41–12.7, p<0.0001).
ConclusionProlonged breastfeeding, regardless of whether it was the only milk an infant drank, greatly increased the risk of egg sensitization. Interestingly, a delayed introduction to whole cow's milk was associated with a reduced possibility of becoming sensitized to eggs. Further studies are required to elucidate these findings.
Globally, the prevalence of food allergy among children can reach up to 8%.1 The foods that commonly produce allergic symptoms within the pediatric population are cow's milk and chicken eggs.2 The frequency at which egg allergies occur is estimated to be 0.5–2.6%3,4; thus, the frequency of sensitization to their proteins can reach up to 20%.5–7
To date, the factors that are associated with egg sensitization have not been fully identified, especially regarding children that are at a high risk of sensitization. It has been suggested that being around house pets, primarily dogs, might result in the reduced probability of egg sensitization8,9; additionally, having siblings and the early introduction of solid foods while children are still being breastfed may also be factors that directly affect food sensitization.8,10
Notably, only some children with allergic diseases manifest sensitization to chicken eggs, while others do not. The aim of this study was to evaluate whether prolonged breastfeeding and the delayed introduction of whole cow's milk into the diet of an infant are associated with egg sensitization.
Methods and patientsEthicsThis study was approved by the ethics committee and the attending hospital's research committee. Since the data were collected directly from patients’ medical records, no written informed consent was required. The confidentiality of the data collected was maintained at all times.
Patient selectionWe performed a matched case–control study, categorized by age and sex, within a teaching hospital. We studied children aged between 2 and 5, all of whom were referred to the allergy department after being diagnosed with an allergic disease (asthma, allergic rhinitis, or atopic dermatitis). All of these children reside within the metropolitan region of Guadalajara, Jalisco; the selection process for this study was carried out between January, 2012 and December, 2013.
The cases and controls were defined by the presence or absence of sensitization to chicken eggs, respectively.
The criteria for matching the controls to the cases were sex and age during the selection process (±1 year) for each candidate.
Both the cases and the controls had a positive cutaneous reaction to at least one of the following allergens: Dermatophagoides pteronyssinus, Dermatophagoides farinae, cockroach mix (German and American cockroach), cat and dog dander, grass pollens (Phleum pratense, Cynodon dactylon), weeds (Chenopodium álbum, Ambrosia trífida, Salsola pestifer, Amaranthus palmeri, Helianthus annus, Artemisia vulgaris), trees (Quercus sp., Fraxinus sp., Prosopis sp., Populus sp., Ligustrum sp., Eucalyptus sp., Alnus sp., Betula sp., Schinus molle), or fungus spores (Cladosporium spp., Alternaria alternata, Caphalosporium sp.).
Data collectionAn instrument for data collection was designed ad hoc in order to compile our data, and each participant's medical history was reviewed. Their medical charts included: family history of atopy, breastfeeding history (duration of a diet exclusive to breastfeeding and the total duration of breastfeeding), the age of introduction to a complementary diet, and the characteristics of this diet, including the introduction of cow's milk. If some of these data were missing in a patient's medical record, we contacted the child's parent and obtained the information via a telephone interview.
Skin prick test techniqueSkin-prick tests were carried out during hospital visits; we used non-standardized allergens at a concentration of 1:20 (p/v) (Allergomex, Mexico). Histamine and glycerin served as positive and negative controls, respectively. The patient was considered to have a positive reaction if the wheal diameter was >3mm in relation to the negative control.11
DefinitionsThe presence of a positive skin-prick test to either egg white or egg yolk determined sensitization to chicken egg allergen.
With regard to the diagnosis of allergic diseases, asthma, allergic rhinitis and atopic dermatitis, the physicians in charge of treating the children carried these out, and data were obtained from the patients’ clinical records.
The presence of asthma, allergic rhinitis, or atopic dermatitis in either the mother or the father determined whether there was a family history of atopic diseases.
Exclusive breastfeeding was defined as the period in which infants only ingested breastmilk (with the exclusion of even water); we made an exception where oral rehydrating solutions, vitamins, minerals or medication in the form of drops or syrups12 were ingested. The age at which a complementary diet was introduced refers to the period when foods other than breastmilk were administered.13
Statistical analysisTo characterize the groups’ cases (sensitization to chicken egg proteins) and controls (no sensitization to chicken egg protein), we determined the frequency and proportion of categorical variables for each group; we also obtained the mean and standard deviation or the median and interquartile range (IQR) of the continuous variables. To compare all proportions, we employed the chi-square or Fisher's exact tests when subgroups with a small sample size were compared. The continuous variables, per their distribution, were compared using Student's t-test or Mann–Whitney U test for two independent samples. Finally, all risk factors that showed significant association in univariate analysis were analyzed using a multivariate model that included the following independent variables: a paternal or maternal history of atopy (one or both; yes or no), duration of breastfeeding (≥6 months vs. <6 months), the age at which solid foods (≥4 months vs. <4 months), whole cow's milk (≥6 months vs. <6 months) and fish or seafood (≥24 months or <24 months) were introduced into the child's habitual nutrition, and coexistence with dogs (yes or no). The child's sensitization to chicken egg proteins (yes or no) was included as a dependent variable. For all of the analyzed variables, the last category was taken as the reference category (OR=1). Our calculations were obtained using multivariate analysis with binary logistic regression, by means of the “Enter” and “Forward conditional” methods. To process this information, we used IBM SPSS 20.0 software (IBM Co., Armonk, NY, USA).
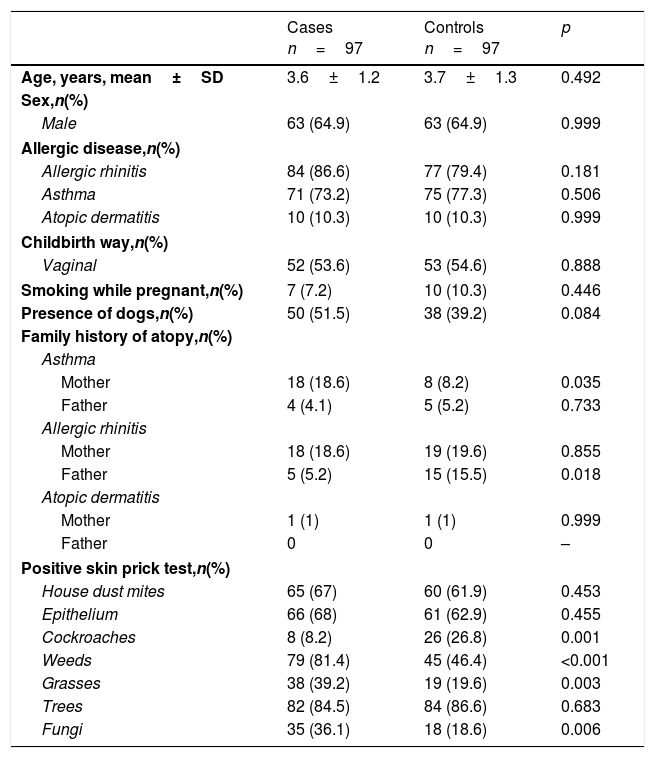
ResultsWe recruited 97 matched cases with 97 controls. In each group, we observed predominance of allergic diseases among boys, as shown in Table 1. The most frequent type of allergic disease in both groups was allergic rhinitis, followed by asthma. There were no significant differences between the cases and controls regarding these diseases. A maternal history of asthma and sensitization to weeds, grasses and fungi were positively associated with sensitization to eggs, while a history of paternal allergic rhinitis and sensitization to cockroaches were inversely associated.
Characteristics of the study groups.
| Cases n=97 | Controls n=97 | p | |
|---|---|---|---|
| Age, years, mean±SD | 3.6±1.2 | 3.7±1.3 | 0.492 |
| Sex,n(%) | |||
| Male | 63 (64.9) | 63 (64.9) | 0.999 |
| Allergic disease,n(%) | |||
| Allergic rhinitis | 84 (86.6) | 77 (79.4) | 0.181 |
| Asthma | 71 (73.2) | 75 (77.3) | 0.506 |
| Atopic dermatitis | 10 (10.3) | 10 (10.3) | 0.999 |
| Childbirth way,n(%) | |||
| Vaginal | 52 (53.6) | 53 (54.6) | 0.888 |
| Smoking while pregnant,n(%) | 7 (7.2) | 10 (10.3) | 0.446 |
| Presence of dogs,n(%) | 50 (51.5) | 38 (39.2) | 0.084 |
| Family history of atopy,n(%) | |||
| Asthma | |||
| Mother | 18 (18.6) | 8 (8.2) | 0.035 |
| Father | 4 (4.1) | 5 (5.2) | 0.733 |
| Allergic rhinitis | |||
| Mother | 18 (18.6) | 19 (19.6) | 0.855 |
| Father | 5 (5.2) | 15 (15.5) | 0.018 |
| Atopic dermatitis | |||
| Mother | 1 (1) | 1 (1) | 0.999 |
| Father | 0 | 0 | – |
| Positive skin prick test,n(%) | |||
| House dust mites | 65 (67) | 60 (61.9) | 0.453 |
| Epithelium | 66 (68) | 61 (62.9) | 0.455 |
| Cockroaches | 8 (8.2) | 26 (26.8) | 0.001 |
| Weeds | 79 (81.4) | 45 (46.4) | <0.001 |
| Grasses | 38 (39.2) | 19 (19.6) | 0.003 |
| Trees | 82 (84.5) | 84 (86.6) | 0.683 |
| Fungi | 35 (36.1) | 18 (18.6) | 0.006 |
SD: standard deviation.
The value of p obtained by Chi square or Student t test depending on its need.
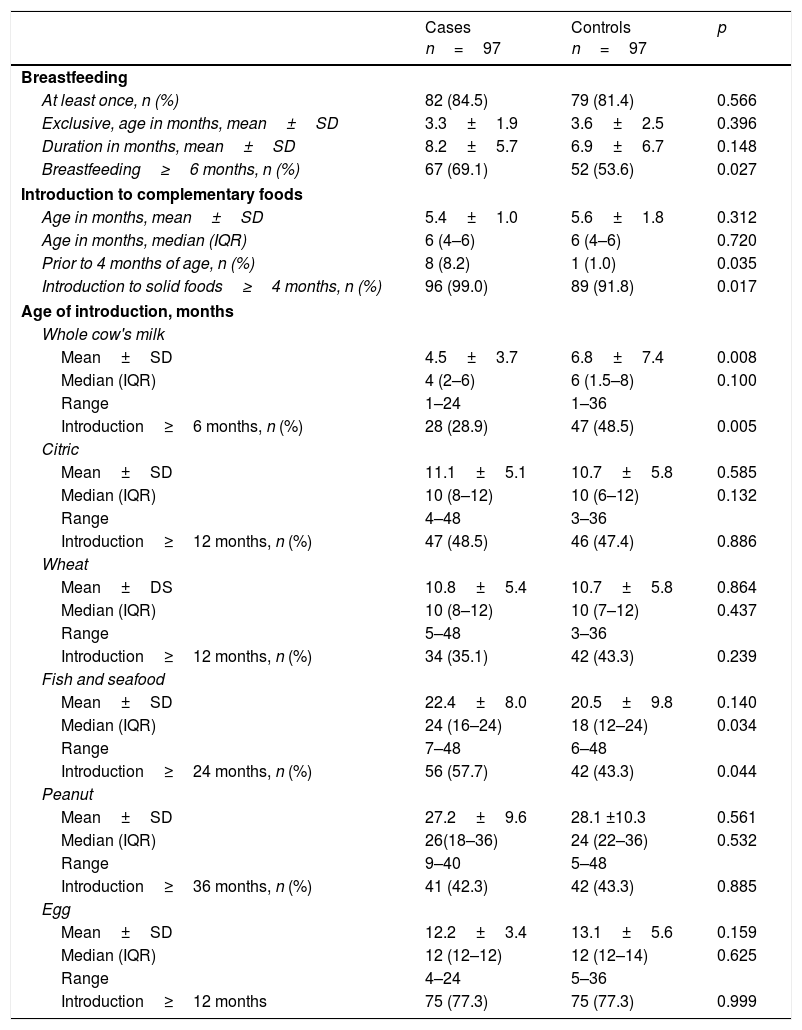
The characteristics of breastfeeding in both study groups were very similar. In that sense, the median age at which the introduction of diverse foods occurred was not associated with sensitization to eggs. However, the frequency of introduction of complementary foods prior to four months of age was significantly higher among those that displayed sensitization to eggs (p=0.035, Table 2).
Nutritional characteristics of the study population.
| Cases n=97 | Controls n=97 | p | |
|---|---|---|---|
| Breastfeeding | |||
| At least once, n (%) | 82 (84.5) | 79 (81.4) | 0.566 |
| Exclusive, age in months, mean±SD | 3.3±1.9 | 3.6±2.5 | 0.396 |
| Duration in months, mean±SD | 8.2±5.7 | 6.9±6.7 | 0.148 |
| Breastfeeding≥6 months, n (%) | 67 (69.1) | 52 (53.6) | 0.027 |
| Introduction to complementary foods | |||
| Age in months, mean±SD | 5.4±1.0 | 5.6±1.8 | 0.312 |
| Age in months, median (IQR) | 6 (4–6) | 6 (4–6) | 0.720 |
| Prior to 4 months of age, n (%) | 8 (8.2) | 1 (1.0) | 0.035 |
| Introduction to solid foods≥4 months, n (%) | 96 (99.0) | 89 (91.8) | 0.017 |
| Age of introduction, months | |||
| Whole cow's milk | |||
| Mean±SD | 4.5±3.7 | 6.8±7.4 | 0.008 |
| Median (IQR) | 4 (2–6) | 6 (1.5–8) | 0.100 |
| Range | 1–24 | 1–36 | |
| Introduction≥6 months, n (%) | 28 (28.9) | 47 (48.5) | 0.005 |
| Citric | |||
| Mean±SD | 11.1±5.1 | 10.7±5.8 | 0.585 |
| Median (IQR) | 10 (8–12) | 10 (6–12) | 0.132 |
| Range | 4–48 | 3–36 | |
| Introduction≥12 months, n (%) | 47 (48.5) | 46 (47.4) | 0.886 |
| Wheat | |||
| Mean±DS | 10.8±5.4 | 10.7±5.8 | 0.864 |
| Median (IQR) | 10 (8–12) | 10 (7–12) | 0.437 |
| Range | 5–48 | 3–36 | |
| Introduction≥12 months, n (%) | 34 (35.1) | 42 (43.3) | 0.239 |
| Fish and seafood | |||
| Mean±SD | 22.4±8.0 | 20.5±9.8 | 0.140 |
| Median (IQR) | 24 (16–24) | 18 (12–24) | 0.034 |
| Range | 7–48 | 6–48 | |
| Introduction≥24 months, n (%) | 56 (57.7) | 42 (43.3) | 0.044 |
| Peanut | |||
| Mean±SD | 27.2±9.6 | 28.1 ±10.3 | 0.561 |
| Median (IQR) | 26(18–36) | 24 (22–36) | 0.532 |
| Range | 9–40 | 5–48 | |
| Introduction≥36 months, n (%) | 41 (42.3) | 42 (43.3) | 0.885 |
| Egg | |||
| Mean±SD | 12.2±3.4 | 13.1±5.6 | 0.159 |
| Median (IQR) | 12 (12–12) | 12 (12–14) | 0.625 |
| Range | 4–24 | 5–36 | |
| Introduction≥12 months | 75 (77.3) | 75 (77.3) | 0.999 |
The value of p obtained by the chi square test, Student t test or Mann-Whitney U test when deemed necessary.
SD: standard deviation.
IQR: interquartile range.
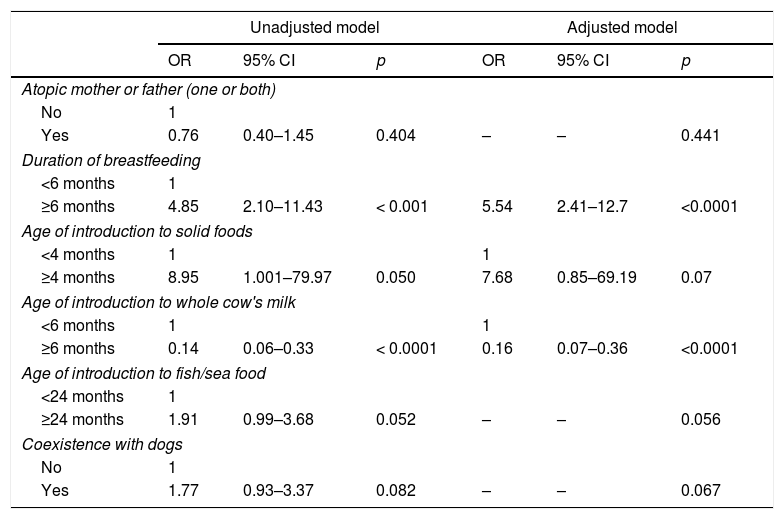
Two variables were identified as factors associated with egg sensitization in the adjusted model. Introduction to whole cow's milk at greater than or equal to six months of age was identified as a statistically significant protective factor (OR=0.16), while a duration of breastfeeding greater than or equal to six months was identified as a statistically significant risk factor (OR=5.54). The age of introduction to solid foods did not leave the adjusted model (OR=7.68) and showed no significant association with egg sensitization (p=0.07). The rest of the variables were excluded from the adjusted model (Table 3).
Factors associated to egg protein sensitization.
| Unadjusted model | Adjusted model | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Atopic mother or father (one or both) | ||||||
| No | 1 | |||||
| Yes | 0.76 | 0.40–1.45 | 0.404 | – | – | 0.441 |
| Duration of breastfeeding | ||||||
| <6 months | 1 | |||||
| ≥6 months | 4.85 | 2.10–11.43 | < 0.001 | 5.54 | 2.41–12.7 | <0.0001 |
| Age of introduction to solid foods | ||||||
| <4 months | 1 | 1 | ||||
| ≥4 months | 8.95 | 1.001–79.97 | 0.050 | 7.68 | 0.85–69.19 | 0.07 |
| Age of introduction to whole cow's milk | ||||||
| <6 months | 1 | 1 | ||||
| ≥6 months | 0.14 | 0.06–0.33 | < 0.0001 | 0.16 | 0.07–0.36 | <0.0001 |
| Age of introduction to fish/sea food | ||||||
| <24 months | 1 | |||||
| ≥24 months | 1.91 | 0.99–3.68 | 0.052 | – | – | 0.056 |
| Coexistence with dogs | ||||||
| No | 1 | |||||
| Yes | 1.77 | 0.93–3.37 | 0.082 | – | – | 0.067 |
OR: odds ratio obtained by binary logistic regression; CI: confidence interval.
Model adjusted by forward conditional method.
Our results suggest that a breastfeeding duration equal to or greater than six months increases the odds of egg protein sensitization; interestingly, the late introduction of whole cow's milk significantly decreased the risk of sensitization to these proteins.
The role that maternal breastfeeding plays in allergen sensitization has not been fully explored, particularly regarding the role that it plays in allergic diseases. However, in both cases, the results obtained thus far have been contradictory. In our study, breastfeeding infants for at least six months, even among high-risk children, was a significant factor contributing to egg sensitization; this factor was independent of whether the infant drank only breastmilk. This result is interesting because it is consistent with previous longitudinal studies that have stated that there is no evidence to suggest that breastmilk helps prevent allergen sensitization. For example, Sears et al. evaluated a group of individuals from the time they were three years of age until they reached their 26th year, and it was concluded that breastfeeding not only offered no protection against sensitization to allergens but actually increased the chances of sensitization.14 In another cohort, Matheson et al. had similar findings, which pointed to breastfeeding being a contributing factor to animal and pollen sensitization.15 Notably, neither of these studies specifically evaluated sensitization to eggs.
Another possible reason for this unexpected association is reverse causality. In this situation, a previous atopic disease might prolong the duration of breastfeeding.16 If this had occurred during this study, our results would give the statistical appearance that prolonged breastfeeding is a risk factor for egg sensitization. Retrospective designs such as case–control studies often present this condition when there is no certainty that the cause occurs before the event. Biases such as memory or information can generate reverse causality.
On the other hand, the early dietary introduction of eggs in children younger than 12 months of age has also been associated with a reduced risk of egg sensitization.17,18 However, in our study, this consideration does not apply because in both of our groups, eggs were introduced to the child's diet after 12 months of age.
With regard to other types of foods, this study found that children with allergic diseases who received whole cow's milk at six months of age or older were at a lower risk of developing egg sensitization. It is likely that the early introduction to whole cow's milk also acts as an agent that facilitates egg protein transference to the child. Recent evidence shows that the early incorporation of whole cow's milk into a child's diet, especially before the child is one month old, reduces the risk of becoming sensitive to its proteins19,20; it would seem that the same could be said about foods such as fish,21 wheat, rice, oats, or barley.22
Regarding children that are breastfed and the moment that solid foods are introduced into their diets, it seems that there is a contradictory effect about allergic diseases and allergy sensitization. We observed that when solid foods were incorporated into a child's diet at three months of age or older, some protection was offered against egg sensitization. In a cohort study from The Wayne County Health Environment, the Allergy and Asthma Longitudinal Study, there were findings that differed from our own; they found that the introduction of solid foods after one month of life was associated with a lower risk of food sensitization.23 In an additional cohort study, The Finnish Type 1 Diabetes Prediction and Prevention project, it was evident that the early introduction of solid foods was associated with a lower probability of allergen sensitization. In regard to eggs specifically, this association was maintained when they were ingested prior to 11 months of age; however, the study also showed that there was greater food diversity at three months of age, which was linked to a higher possibility of allergen sensitization.22 In an important epidemiological study, The Health Nuts report, the introduction of baked eggs at four to six months of age protected against the development of an egg allergy.18 Thus, it seems that the early introduction of solid foods among infants that are still being breastfed could potentially contribute to regulating immunity, which would reduce the manifestation of allergic diseases and allergen sensitization.
For egg allergy, it is expected that over the years, most children's allergies will spontaneously resolve. The Boyano-Martinez et al. study found that in a group of children allergic to eggs, 50% overcame their allergy after they continued to ingest them for 35 months; after the age of five, the termination of the allergy increased to 66%.24 Furthermore, an additional study carried out in children with atopic dermatitis showed that 41% had overcome their egg allergy by the age of three, 60% at the age of five, and 85% at the age of 10.25 Recently, a multi-center study had similar findings; 50% of the children in their study group overcame egg allergies by 72 months of age.26 Moreover, a population-based study showed that 47% of children allergic to egg had resolved their allergies by the age of two.27 There are several factors that have been found to influence the resolution of the egg allergy, including the following: the basal IgE serum levels against egg proteins, the basal clinical characteristics of this reaction, frequent ingestion of baked eggs, the size of the papule that appears after a skin-prick test, and the early detection of an egg allergy.4,24–27
Some of the studies carried out around the world have shown that pet dogs offer an apparent protection against egg sensitization. For example, The Health Nuts Study, a population-based project in Australia, revealed that children who maintained contact with a household dog had a 28% reduced chance of being allergic to eggs.18 The effect that pets have on atopy has primarily been researched in regard to the prevalence of allergic diseases, which has led to conflicting results. Recently, during a meta-analysis that only included cohort studies, it was concluded that exposure to dogs, but not cats, reduces the risk of atopic dermatitis among children.9 In contrast, a study undertaken in China, which included over 16,000 children, found that exposure to dogs was a risk factor for developing asthma.28 When a positive association between dogs and a reduced frequency of allergy sensitization is established, it is attributed to endotoxins29; a dog's (but not a cat's) bacterial diversity could also act as a modulating agent on the immune response.30 We chose to evaluate the role that dog interactions might play with regard to egg sensitization, since it has been attributed to reducing the risk of egg sensitization among children with atopic diseases. However, we did not find any association between the two; thus, this matter remains to be fully understood. In our study, a family history of atopy did not increase the risk of developing egg sensitization; like breastfeeding, the role that atopy plays in allergen sensitization has been less studied than the role it plays among allergic diseases.
This study does have some limitations that need to be taken into account when interpreting its results. First, we did not consider the amount of whole cow's milk that was administered to children during the first months of their lives. Second, we had a very small sample group of children with atopic dermatitis, which is one of the allergic diseases that seem to be closely related to food allergies. Regarding the recall bias of case–control studies, we believe that recall bias did not occur in this study because the data were obtained from the patients’ clinical histories; the parents did not have to perform a recall exercise. Unlike previous studies, another important limitation in our study was that we evaluated the sensitization process and not egg allergy specifically; thus, we recommend caution when interpreting these results because the factors associated with egg protein sensitization may differ from those associated with egg allergy.
In conclusion, the probability of egg sensitization among children with allergic diseases is increased when children are breastfed for the first six months of their lives or longer, and it is reduced when the incorporation of whole cow's milk is delayed for at least the first six months of the infant's life.
FundingNone declared.
Conflicts of interestThe authors have no funding or conflicts of interest to disclose.