Beta-1-3 Glucan is a polysaccharide extracted from Saccharomyces cerevisiae with a possible immunomodulating action that may have a favourable action on asthma symptoms and other allergic diseases. An experimental study carried out using a murine respiratory model detected a decrease in pulmonary tissue eosinophilia, as well as an increase in Interleukin-10 (IL-10) after glucan use.
MethodsThis open, exploratory study with blind outcome evaluation included asthmatic children between 6 and 12 years of age with mild to moderate persistent asthma and inadequate disease control (rescue medication needed more than twice a week) in spite of inhaled budesonide 400μg/day. After a four week run-in period, subcutaneous Beta-1-3-glucan injections were given weekly for the first four weeks and then every two weeks for the last four weeks. IL-10 levels, measured by the immunoenzymatic method (ELISA), were compared before and after glucan administration.
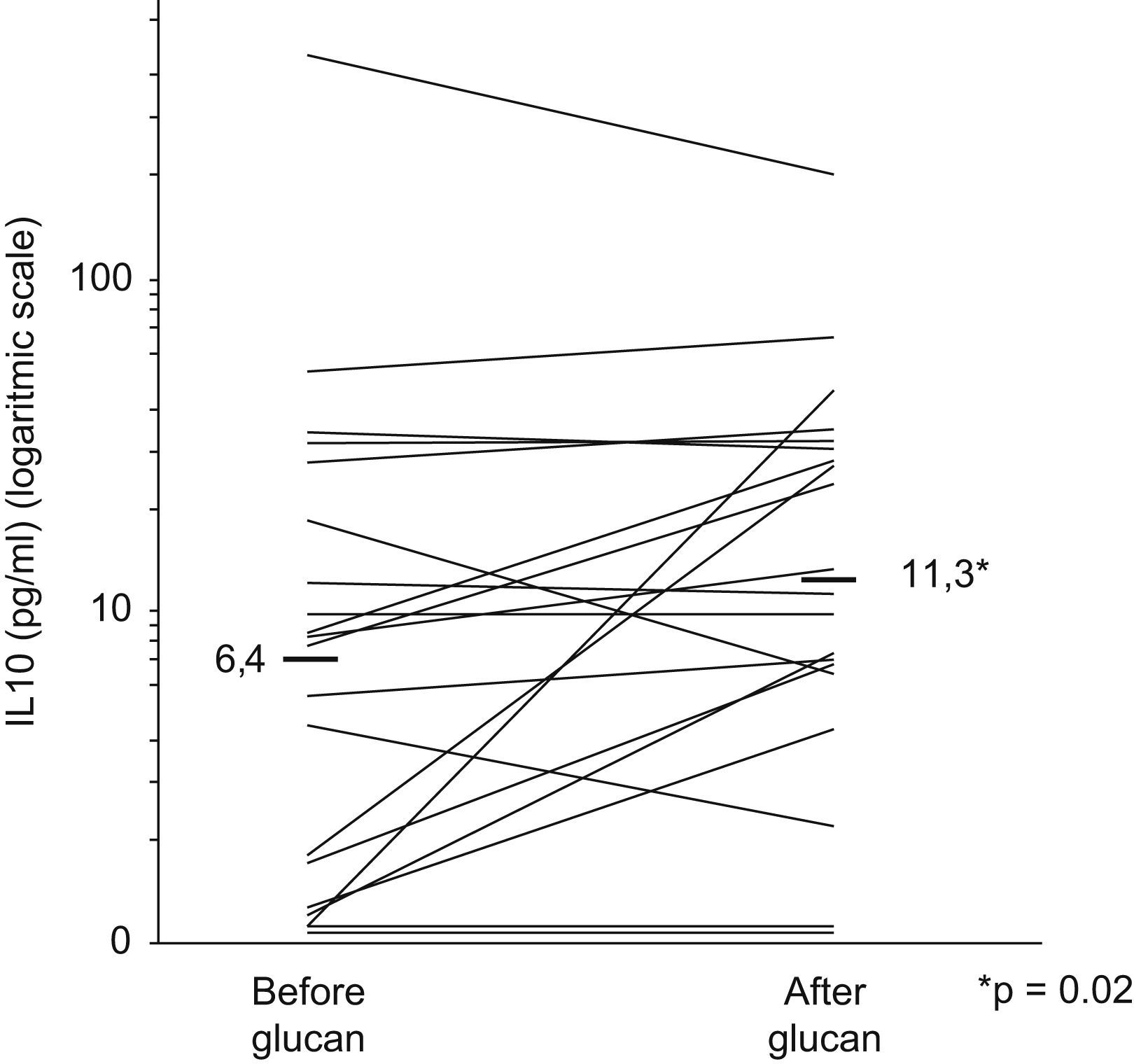
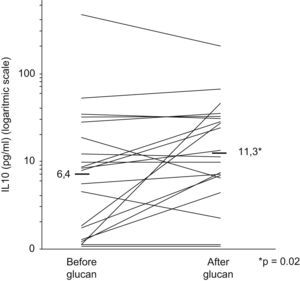
ResultsTwenty patients (14 male and 6 female) were included. Mean IL-10 levels were 6.4pg/ml and 11.3pg/ml before and after glucan, respectively (p=0.02). There was also a reduction of asthmatic symptoms score at the end of study.
ConclusionsThis is the first study which shows that subcutaneous particulate Beta-1-3-glucan increases serum IL-10 levels in asthmatics. The possibility of glucan being able to modulate allergic sensitisation and having a beneficial action in restoring Th2 function should be assessed by means of properly planned controlled clinical trials, as it may represent a new therapeutic strategy.
Allergic diseases, including asthma, have reached epidemic proportions in several countries around the world. Epidemiological studies have shown an inverse association between infections and allergies, an observation that influenced the formulation of the “hygiene hypotheses” and still arouses a great interest in the understanding of the pathogenic mechanism of these diseases.1–4
These diseases are characterized by the development of specific IgE against certain allergens. A new contact with these allergens activates mastocytes and results in inflammation; symptoms due to the release of mediators; and infiltration of mucosa by cells, particularly eosinophils. One of the proposed mechanisms that might facilitate the typical allergic inflammation response is the immune imbalance consequent to Th2 lymphocyte hyperfunction due, among other factors, to a low production of interleukin 10 (IL-10), which results in altered T lymphocyte regulation.5–7
Therapeutic strategies to change the natural history and progress of asthma and other allergic diseases are required. Perhaps drugs able to modulate the immune system could influence the cytokine profile aiming towards decreasing the exaggerated Th2 response of allergic patients and replacing it for an efficient and balanced Th1 response.8–10
In the respiratory and intestinal mucosa, antigen processing and presentation by dendritic cells and/or macrophages is associated with a Th1 immune pattern that can suppress allergic symptoms.11,12 This Th1 immune response may also be triggered by some bacteria and/or their products, such as Beta-1-3-glucan.
Beta-1-3-glucan is a polyglucose from fungi and yeast cells’ walls. As it is not present in animal cells, it is recognized by the immune system as non-self, leading to a widespread stimulus for the innate immune system, a fact that could be explored in restoring human health or even in the prophylaxis of diseases.13
Depending on the origin, there are several kinds of glucan, with different biological actions.14 Beta-1-3-glucan, derived from Saccharomyces cerevisiae, has been prepared and used in basic research and clinical trials for several years now.13 The ability to activate the innate immune response resides in the insoluble fungus wall fraction, known as Zymosan; its main component is Beta-1-3-glucan, a polysaccharide contained in the inner part of yeast cell walls.15,16
An experimental trial carried out in a murine model of respiratory disease has detected a decrease in pulmonary tissue eosinophilia, as well as a functional increase of regulatory T cells and IL-10 after glucan administration,17 a result that may be attributed to macrophage stimulation. Our research was carried out in order to test the hypothesis that the subcutaneous use of Beta-1-3-glucan in mild to moderate asthmatic children could modify interleukin-10 serum levels.
Material and methodsStudy subjectsThe research was approved by the institutional Ethics Committee and all parents or guardians signed the informed consent.
The study was carried out at the Research Center for Allergy and Immunology at Hospital das Clínicas from Universidade Federal de Pernambuco, Recife, Brazil. It included outpatients from 6 to 12 years of age with mild or moderate persistent asthma and inadequately controlled disease (characterized by rescue medication needed more than twice per week) after a four week run-in treatment period of inhaled corticosteroid (budesonide 400μg/day). Thus, patients included were non-controlled asthmatics despite previous treatment with inhaled corticosteroids and none had any change in their prescribed therapeutic scheme during treatment with glucan.
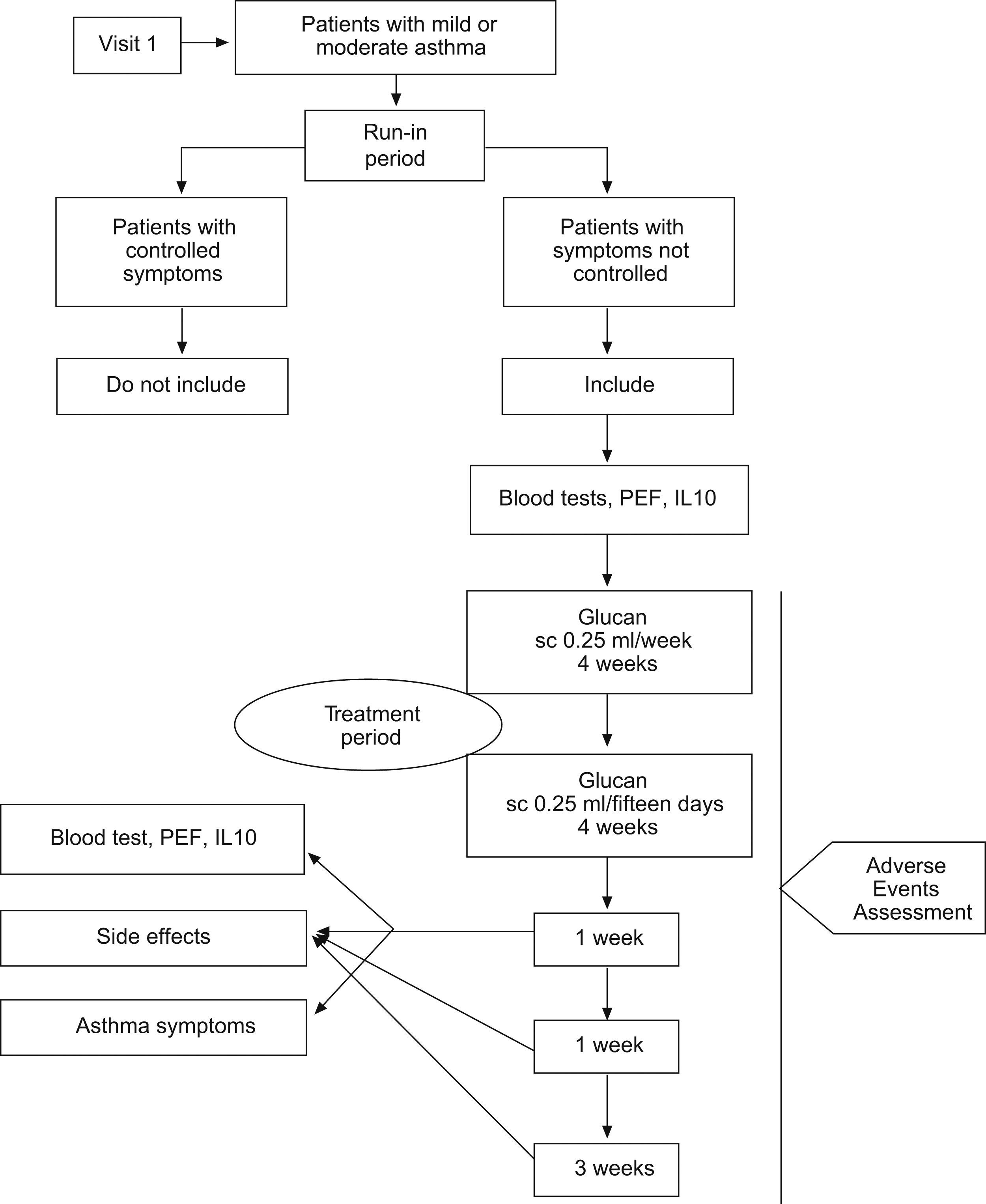
All 20 children had blood exams (total serum IgE, IL-10, haemogram with platelets counts, aspartate and alanine aminotransferases, urea, creatinine) and peak expiratory flow rate (PEF) before glucan treatment period as shown in the research flow diagram (Fig. 1).
Study design and sample estimateThis is an open label study with a blind outcome evaluation (quasi-experimental) designed as an exploratory project to assess changes in IL-10 serum levels after eight weeks treatment with subcutaneous Beta-1-3-glucan in mild to moderate asthmatic children.
For sample estimation we used the work of Ceyhan el al.18 who observed IL-10 serum levels in asthmatics of 4.3±3.8pg/ml. Twenty patients were needed in order to be able to detect a 3.5pg/ml difference after treatment with α and β errors of 0.05 and 0.20 (two-tailed) respectively.
Treatment with Beta- 1-3-glucan and adverse events monitoringDuring the eight weeks glucan (Imunoglucan®) treatment period patients continued to have their asthma medication. For the first four weeks 0.5mg (0.25ml) of glucan was given by subcutaneous injections once a week; each application was carried out in a different limb in order to assess for any local side effects. For the last four weeks the interval between injections was increased to every two weeks. At each new visit the patient was examined before receiving the glucan dose in order to check for hyperaemia, pain, nodulation or abscess on the injection area.
Parents or guardians filled a diary of asthma symptoms (Visual Analogue Scale) and use of rescue medication (salbutamol sulphate canister consumption). Side effects such as fever, headache, asthenia, myalgia and hyperaemia, pain, nodulation or abscess at injection site or any other complaints were also recorded, as well as morning and bedtime peak expiratory flow rate. One week after the last injection, blood samples were drawn for IL-10 dosage and to repeat blood tests. The follow-up period continued for the ensuing three weeks in order to assess whether there were any side effects after medication was stopped.
Interleukin-10 dosageSerum was separated from whole peripheral blood by centrifugation and stored at −20°C in coded vials. IL-10 determinations were blindly done and concealment was achieved as the researcher was not able to identify the patients or either if the sample was from before or after treatment. IL-10 was quantified by means of the immunoenzymatic method (ELISA) using the IL-10 Human, Biotrak Easy ELISA – RPN5962 commercial kit (Amersham Biosciences UK Limited, Buckinghamshire, UK), all the manufacturer's recommendations were followed and the exam was carried out at the laboratory of Clinical Immunology of the College of Pharmacy of Universidade Federal do Rio Grande do Norte, Brazil. In brief, the technique involved the following: serum samples and standards with known concentrations of IL-10 were placed in duplicates in well plates sensitised with anti-IL-10 human monoclonal antibody and containing biotin coupled anti-IL-10, sample diluent and lyophilised streptavidin-HRP. They were incubated for 3h at 18°C [64.4°F] in a microplate rocker; plates were then washed and the substrate solution (tetramethyl benzidine-TMB) was added. After 15min incubation under agitation 1M phosphoric acid was pipetted in so as to interrupt the reaction. A reading of the calorimetric reaction was taken at 450nm using a microplate reader (Expert Plus-Asys, Eugendorf, Aus) and results expressed in pg/mL.
Statistical analysisAs IL-10 log-transformation showed a fair adjustment to normal distribution, Student's paired t test was carried out to compare means.
The study also had the secondary endpoint of checking for adverse events and changes in symptoms. The symptoms change analysis was done comparing only the 4th week of run-in period to the last week of treatment period by Friedman nonparametric test for paired data.
ResultsThe sample included 20 patients with persistent mild (10 patients) and moderate (10 patients) asthma who were followed up for 12 weeks. There were 14 male and 6 female, with age median of 8 years (6–12 years). The research included only those patients with partly controlled asthma (use of rescue medication more than twice a week) despite the use of 400mcg/day of inhaled budesonide in the first four weeks (run-in period).
As is shown in Fig. 2, mean serum IL-10 rose from 6.4pg/ml to 11.3pg/ml (p=0.02).
The commonest complaint reported by the patients after subcutaneous injection of Beta-1-3-glucan was short-term local pain but there was no report of any painful nodulation or abscess. There was no adverse event demanding treatment interruption or any other intervention.
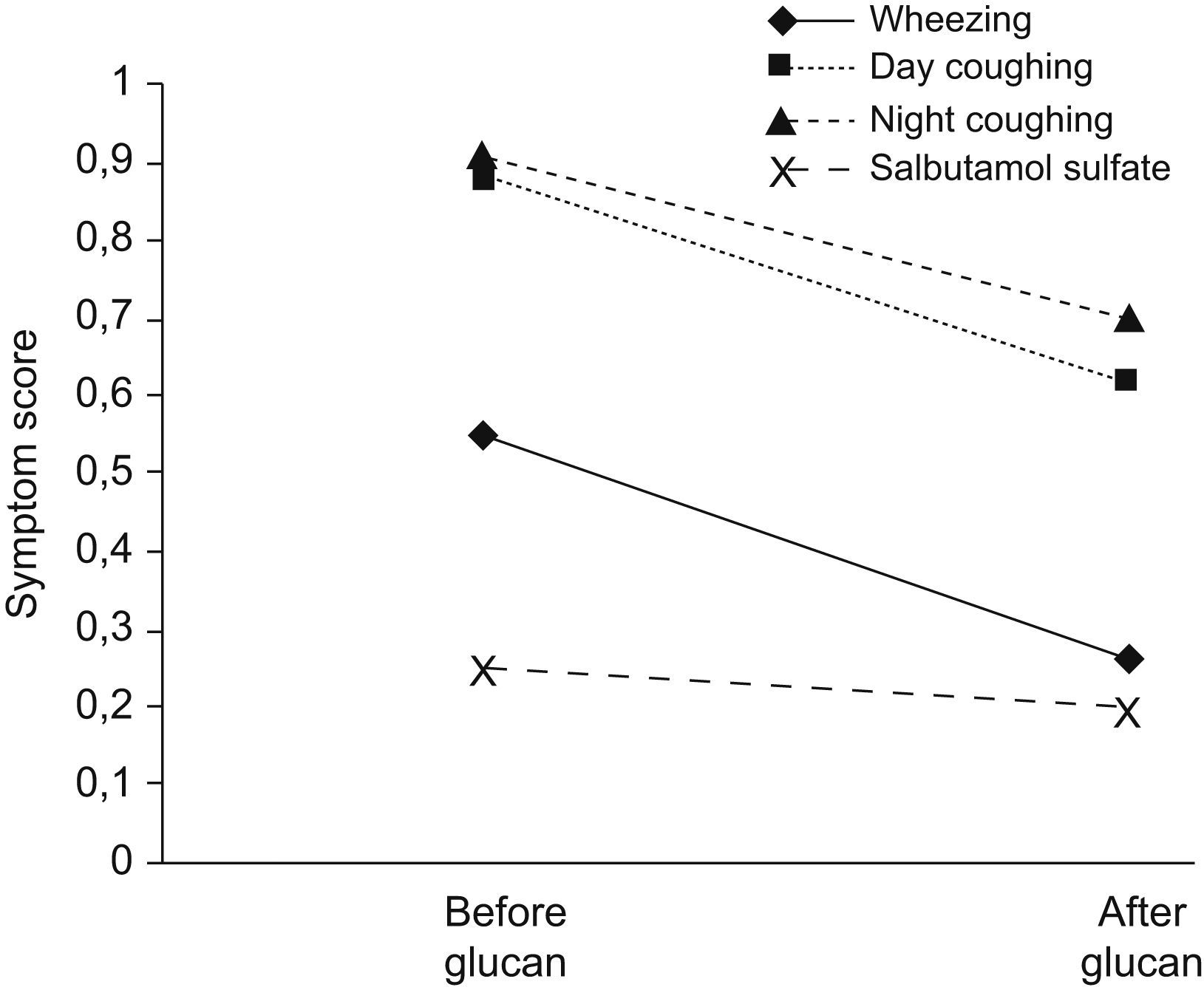
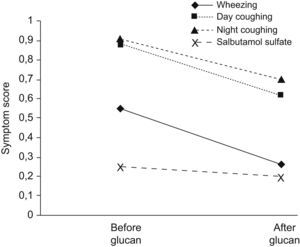
Despite not being designed as a trial to assess clinical treatment response, symptoms showed a significant improvement in diary scores in wheezing and day and night cough but not in salbutamol sulphate rescue use (Fig. 3).
Mean diary symptom score of the fourth week of run-in period and the 12th week of Beta1-3-glucan administration. There was an improvement in wheezing (p=0.022), day coughing (p=0.018), nocturnal coughing (p=0.044) and there was no significant decrease in Salbutamol Sulphate rescue use (p=0.722).
Beta-1-3-glucan is a polyglucose without lateral ramifications. It has no primary toxic action and shows wide immunologic activity since it does not exist in animal cells. Due to its capacity of coupling with the Toll Like Receptor 2 (TLR2), Dectin-1 (type C Lectin) and other receptors, Beta-1-3-glucan is a powerful stimulant of the innate immunologic system and of the phagocytose macrophage defence mechanisms.13,19–22 The particulate form of glucan derived from Saccharomyces cerevisiae was capable of flagging activation of innate immunity directly by means of Dectin-1 without any need to activate reactive linking TLR-2.23 Likewise, it has been shown that Beta-1-3-glucan is capable of stimulating tumoricidal activity of Polymorphonuclears, Macrophages and Natural-Killer Cells, and these effects are mediated by its capacity to link with lecithin in the complementary receptor 3 (CR3), thus making it work as an immunomodulator for several infectious diseases and neoplasias.13,22,24 On the other hand, there are almost no studies on a possible effect of Beta-1-3-glucan on allergic diseases.
Glucan action mechanism is dependent on the way it is administered (oral, venous, intramuscular, subcutaneous) and of some characteristics, including the source, solubility, molecular mass, purity and structural conformation. An improvement in the neutrophyle function, the oxidative burst, bacteria death and increase in nuclear transcription factors after the use of yeast glucan have been reported.25
This study is the first to demonstrate that injectable particulate Beta-1-3-glucan is capable of increasing serum IL-10 levels in asthmatics. Moreover, diary symptoms records showed an improvement in wheezing and day and night cough in these not well-controlled patients in spite of inhaled budesonide 400μg/day.
Asthma inflammatory process is the result of a complex set of innate and acquired immune system interactions. As for innate immunity, the participation of the antigen-presenting cell in the genesis of asthma has been increasingly highlighted, since it is the starting point of Th2 bias immune arrangement. Beta-1-3-glucan has been proving itself to be a medication with a powerful action on interferon-gamma production, in stimulating macrophages and in its differentiation to antigen-presenting cells.13 Macrophages are able of modulating the immune response because they secrete anti-inflammatory mediators such as Prostaglandin E2 (PGE-2), Tumor Growth Factor (TGF-a) and IL-10.13 As such, Beta-1-3-glucan can act as a macrophage stimulant and prevent the appearance of a Th2 response.
In an animal model a single high dose of Beta 1-3-glucan has been related to an improvement in asthma and pulmonary function abnormalities.17 Likewise, it has been reported that intradomiciliary exposure to high levels-concentration above 60μg/g-of 1–3-β-D-glucan was associated with a low risk of wheezing in infants born from atopic parents; this effect was more emphatic in allergen sensitised children.26
In a clinical trial using oral glucan, Yamada et al.27 have found that patients with allergic rhinitis showed an improvement in seasonal and perennial symptoms after the use of that medication. Besides, some patients continued to show improved symptoms up to 6 months after the treatment was interrupted, which might suggest the effect was sustained.27
An improvement in oxidative burst, increase in nuclear transcription factors and in neutrophil and macrophage functions have been reported after the use of particulate glucan from Saccharomyces cerevisiae,28 a fact that had not been described with the orally-administered soluble glucan.
Despite our study's design not being adequate to assess an improvement in asthma symptoms and the small patient number, there was a symptom improvement. This may be due to a placebo effect, but the increase of IL-10 in these patients’ serum may point to the possibility of a therapeutic effect of the medication. The possibility that Glucan may favourably interfere on allergic diseases and exert a beneficial action in restoring Th2 function, modulating allergic sensitisation, should be assessed by means of properly planned and controlled clinical trials, as it may become a new therapeutic strategy.