The aim was to evaluate the impact of Cupressus sempervirens (Cs) and Juniperus communis (Jc) sensitisations in “Maremma” in southern Tuscany.
Methods811 consecutive outpatients (357 F – 57.86%; age: 36.9±16.6) with suspected allergic respiratory symptoms underwent skin prick tests (SPT) for common allergens and for Cs and Jc.
ResultsSPT resulted negative in 295 (36.37%) subjects. A Cs/Jc sensitisation was found in 294 (36.25%): 289 (98.3%) were sensitised to Cs whereas 198 (67.34%) to Jc. There was a co-sensitisation between Cs and Jc in 193 (65.6%) subjects. Cs/Jc mono-sensitisation was found in 39 (13.6%) subjects. A higher number (p<0.0001) of Cs/Jc sensitised subjects reported winter (131–44.55%) and spring (124–42.2%) symptoms compared to Cs/Jc non-sensitised and non-allergic subjects. Most Cs/Jc sensitised subjects reported rhinitis and conjunctivitis (p<0.0001), whereas only few reported coughing and asthma (p<0.01). The most frequent co-sensitisation was with grass, olive and other trees in Cs/Jc subjects (p<0.001). Those who reported winter symptoms, likely influenced by Cupressaceae, rhinitis was the main symptom whereas asthma was less frequent. Cs/Jc sensitisation resulted to be a risk factor (OR: 1.73 [CI95% 1.18–2.55]) for rhinitis whereas the probability of being asthmatic was reduced (OR: 0.62 [CI95% 0.44–0.85]).
ConclusionThe prevalence of Cs/Jc sensitisation is about 36% in “Maremma”. However, only in 44% of the patients, Cs/Jc seem to cause typical winter symptoms. Rhinitis is the predominant symptom, whereas asthma is less frequent. Testing Cupressaceae sensitisation using Jc pollen extract, rather than Cs, may result to be less sensitive.
In recent years the Cupressaceae family has been accepted as an important cause of pollen allergy.1 The most common species around the Mediterranean basin and particularly in Italy, are Cupressus sempervirens, Cupressus arizonica and Juniperus communis. High rates of cross-reactivity within the Cupressaceae family have been detected by means of in vivo and in vitro testing.1 Cupressaceae pollination is generally in winter between January and March, but in October–December and in April it is also possible to measure a significant airborne pollen concentration.2,3 Considerable variations in the dates and in the maximum pollination can occur from year to year depending on species, countries and climate changes.3–6 Cypress pollinosis is characterised by allergic symptoms such as conjunctivitis, rhinitis, dry cough and asthma, with the first three being more frequent, whereas the last is less frequent.1,7,8 Many reports suggest that Cupressaceae pollen has become an increasing cause of respiratory allergic diseases, particularly in European Mediterranean areas1 even though a recent European multicentre SPT survey does not confirm this trend.9 The prevalence of cypress pollen sensitisation in the general population increased from 2.4% to 3.6% between the beginning and the end of the nineties.10–12 A large increase in sensitisation to C. sempervirens pollen in outpatients with suspected allergic respiratory symptoms was also observed in Italy between the beginning and the end of the nineties (from 7.2–9.3% to 22–30.4%).4,12–15 In 2006 this prevalence increased further to 65% in Apulia in southern Italy.8 This is probably due to a considerable rise in the levels of airborne pollen produced by the overuse of these plants for ornamental purposes, or merely due to a better quality of the extracts used in recent years.1
No research regarding this topic, in particular on the sensitisation to J. communis, had been previously carried out in Tuscan “Maremma”. Furthermore, there are few data regarding the real clinical impact of this allergy. In fact, multi-sensitised subjects with Cupressaceae sensitisation do not always report typical winter symptoms,8 probably due to a cross-reactivity rather than a co-sensitisation between allergen components from both taxonomically related and unrelated pollen families.16–18
“Maremma” is an area located in southern Tuscany in central Italy and the Cupressaceae family (in particular C. sempervirens and J. communis) represents a landscape and cultural peculiarity of this area due to a remarkable distribution of these plants, mainly used for ornamental purposes. There are no definite data about the distribution of these plants in “Maremma” but in accordance with a national Italian forest census, the Tuscany area has a higher distribution of Cypress formations in forests compared to other regions19: in 1,100,000 hectares of forest surface, approximately 4400 hectares are represented by Cypress, excluding the large presence of these trees along avenues and roads, in parks and in gardens both in the town and in the countryside that characterise the Tuscan and “Maremma” landscape and which are probably the major cause of respiratory allergy. Other Italian central regions (Marche, Umbria) also show a high presence of Cypress. On the contrary, there is no presence of Cypress in forests in the northern regions (Piemonte, Valle d’Aosta, Trentino alto Adige, Veneto, Lombardia, Friuli Venezia Giulia),19 probably due to the different climatic conditions. In the southern regions the situation varies, with areas without Cypress forests (Molise, Basilicata) and others such as Sicily with a higher distribution of Cypress, due to a recent reforestation policy.19 The different distribution of these plants in the various zones in Italy seems to reflect the results of a multicentre Italian study where the Cupressaceae sensitisation prevalence was different in northern (9.2%), central (28.2%) and southern Italy (20.1%).7
J. communis is a plant belonging to Cupressaceae family and it is common in “Maremma” in the hills and coastal areas20 and flowers between February and May–June. There are no data on the prevalence of sensitisation to this plant.
The aim of this study was to evaluate the prevalence and the real clinical impact of the allergy to C. sempervirens (Cs) and J. communis (Jc) through skin prick tests in outpatients with suspected allergic respiratory symptoms residing in Tuscan “Maremma”, where the distribution of these plants is high.
Materials and methodsEight hundred and eleven consecutive subjects (448 F – 55.24%; age: 37±16.48), who came to our outpatient pulmonary clinic between 2007 and 2010 – with complaints of upper (rhinitis) and/or lower respiratory (dyspnoea, coughing and wheezing) tract disorders and/or conjunctivitis and who supposedly suffered from a respiratory allergy – were considered as candidates. All subjects underwent skin prick tests (SPT) for common aeroallergens (ALK Abelló, Lainate, Milan) using a panel containing the following allergen extracts: grasses (cocksfoot, meadow fescue, rye grass, meadow grass, timothy), pellitory, trees (olive, birch, poplar, plane), mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae), moulds (Alternaria, Aspergillus and Cladosporium species), animal danders (cat, dog, bird and horse) and obviously Cs and Jc (both from Stallergenes, Milan). Positive control (histamine phosphate 10mg/mL) and negative control (glycerol) solutions were applied and the tests were read after 15min. The presence of an erythema of at least 10mm, a wheal 3mm larger than the negative control, was considered as a positive response. All the above-mentioned reported symptoms were taken into consideration. All subjects were asked in which season these symptoms appeared or worsened. Symptoms reported in autumn and winter were considered together because only few subjects reported autumnal symptoms and they often appeared combined with the winter symptoms. Coughing, without dyspnoea and wheezing, was considered as a separate symptom. All data were collected and analysed retrospectively from archive folders. For the purpose of this study, the subjects were subdivided into Cs/Jc sensitised, Cs/Jc non-sensitised and negative SPTs groups. Furthermore, the Cs/Jc sensitised subjects were subdivided into those who reported isolated winter symptoms or a worsening during this season and those who showed symptoms during spring–summer.
Statistical analysisCategorical variables are expressed as percentages. The continuous variable (age) is expressed as mean value, accompanied by its standard deviation. Comparisons between groups were made with the Chi-square test and One-way ANOVA test, where appropriate. The Bonferroni test was used to perform a post-hoc analysis. Subsequently, logistic regression analysis was carried out. The risk of either asthma or rhinitis or asthma plus rhinitis and either conjunctivitis or coughing for all allergies were evaluated using a univariate analysis to explore each variable in the data set. The variables that maintained statistical significance (p<0.10) at univariate analysis were used for multivariate backward analysis.
ResultsSkin prick tests resulted negative in 295 subjects (36.37%). The sensitisation to Cs/Jc resulted positive in 294 patients (36.25%) whereas the other 222 (27.37%) subjects showed a positive SPT to the other allergens. Among all the sensitised subjects, 382 (73.9%) were multi-sensitised. Mono-sensitisation to Cs/Jc was found in 39 patients: 13.26% of Cs/Jc sensitised subjects, 7.54% of all allergic patients. Among these Cs/Jc mono-sensitised subjects, winter symptoms (typical of Cupressaceae allergy) were reported in 69.2% of cases and 87.17%, 23%, 23% and 25.64% highlighted rhinitis, conjunctivitis, coughing and asthma, respectively. There was Cs and Jc co-sensitisation in 193 (65.6%) subjects, whereas 96 (32.65%) showed sensitisation to Cs alone and five (1.7%) to Jc alone.
Perennial symptoms were reported by 437 (53.9%) subjects, whereas in 280 (34.5%), 67 (8.3%) and 278 (34.3%) patients the symptoms appeared or worsened in spring, summer and winter, respectively. A higher number of Cs/Jc non-sensitised subjects reported perennial symptoms compared to Cs/Jc sensitised subjects (p<0.0001). On the contrary, a higher number of Cs/Jc sensitised subjects (131 – 44.55%; 25.3% of all sensitised patients) reported symptoms in winter and in spring (124 – 42.2%; p<0.0001) compared to the other two groups (Table 1).
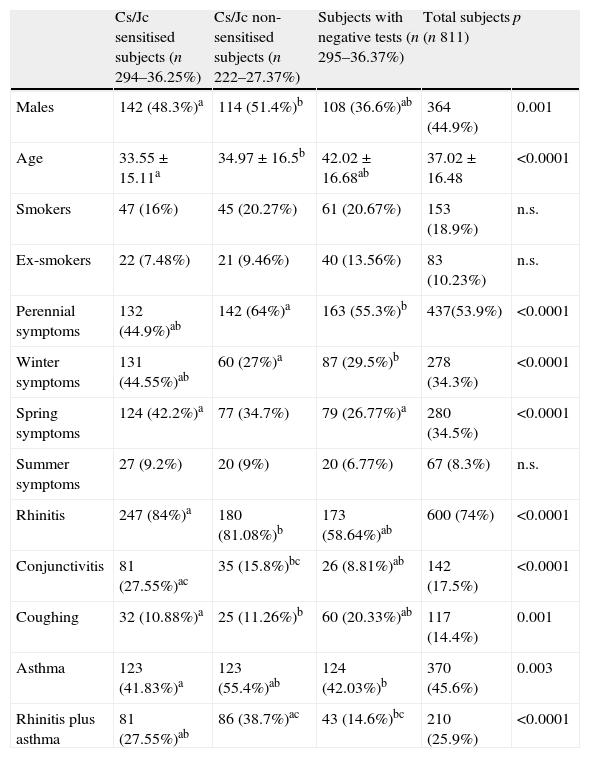
Characteristics of 811 consecutive subjects subdivided into the following three different categories.
| Cs/Jc sensitised subjects (n 294–36.25%) | Cs/Jc non-sensitised subjects (n 222–27.37%) | Subjects with negative tests (n 295–36.37%) | Total subjects (n 811) | p | |
| Males | 142 (48.3%)a | 114 (51.4%)b | 108 (36.6%)ab | 364 (44.9%) | 0.001 |
| Age | 33.55±15.11a | 34.97±16.5b | 42.02±16.68ab | 37.02±16.48 | <0.0001 |
| Smokers | 47 (16%) | 45 (20.27%) | 61 (20.67%) | 153 (18.9%) | n.s. |
| Ex-smokers | 22 (7.48%) | 21 (9.46%) | 40 (13.56%) | 83 (10.23%) | n.s. |
| Perennial symptoms | 132 (44.9%)ab | 142 (64%)a | 163 (55.3%)b | 437(53.9%) | <0.0001 |
| Winter symptoms | 131 (44.55%)ab | 60 (27%)a | 87 (29.5%)b | 278 (34.3%) | <0.0001 |
| Spring symptoms | 124 (42.2%)a | 77 (34.7%) | 79 (26.77%)a | 280 (34.5%) | <0.0001 |
| Summer symptoms | 27 (9.2%) | 20 (9%) | 20 (6.77%) | 67 (8.3%) | n.s. |
| Rhinitis | 247 (84%)a | 180 (81.08%)b | 173 (58.64%)ab | 600 (74%) | <0.0001 |
| Conjunctivitis | 81 (27.55%)ac | 35 (15.8%)bc | 26 (8.81%)ab | 142 (17.5%) | <0.0001 |
| Coughing | 32 (10.88%)a | 25 (11.26%)b | 60 (20.33%)ab | 117 (14.4%) | 0.001 |
| Asthma | 123 (41.83%)a | 123 (55.4%)ab | 124 (42.03%)b | 370 (45.6%) | 0.003 |
| Rhinitis plus asthma | 81 (27.55%)ab | 86 (38.7%)ac | 43 (14.6%)bc | 210 (25.9%) | <0.0001 |
Cs: Cupressus sempervirens; Jc: Juniperus communis.
Winter symptoms: symptoms reported from October to March; subjects that reported a worsening of symptoms in winter or had illnesses only in this season were considered.
Coughing, without dyspnoea and wheezing, was considered as a separate symptom.
Comparisons were made with χ2 and ANOVA tests. (post-hoc analysis with Bonferroni test); abcstatistically significant comparisons.
When we considered all sensitised patients, rhinitis was reported by 600 (74%) subjects, whereas 370 (45.6%), 142 (17.5%) and 117 (14.4%) subjects reported asthma, conjunctivitis and coughing, respectively (Table 1). Rhinitis was the most common symptom reported by all sensitised subjects (Cs/Jc sensitised and Cs/Jc non-sensitised) compared to non-sensitised subjects (p<0.0001; Table 1). Asthma and conjunctivitis were the most frequent symptoms in Cs/Jc non-sensitised patients (p=0.003) and in Cs/Jc sensitised subjects (p<0.0001). Coughing was the most common symptom in subjects with a negative SPT (p=0.001) (Table 1).
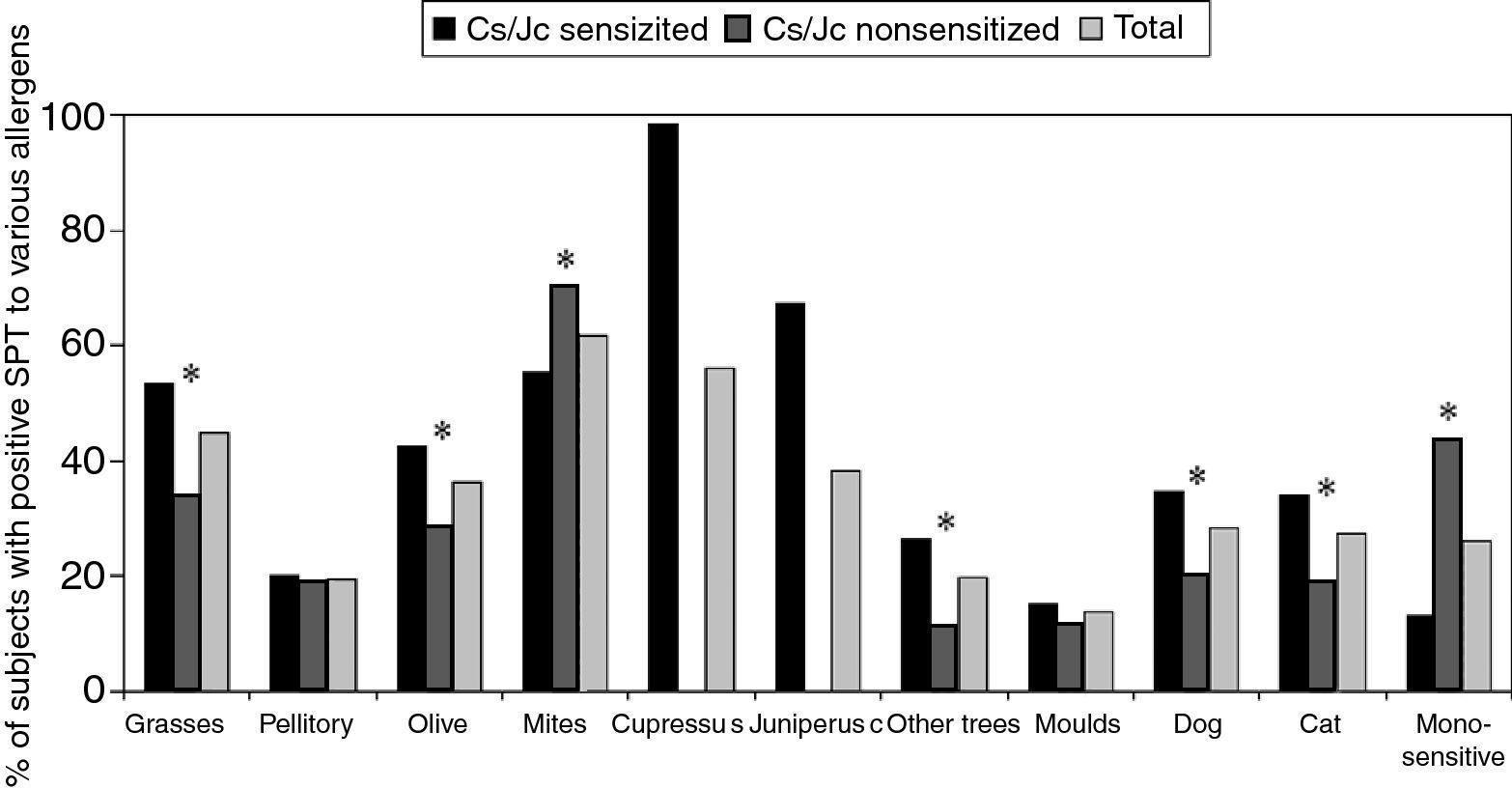
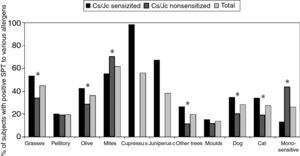
The prevalence of sensitisation to various allergens tested in our study was the following: 319 (61.8%) subjects were positive to house dust mite, 289 (56%) to Cs, 198 (38.4%) to Jc, 231 (44.8%) to grasses, 188 (36.4%) to olive tree, 101 (19.6%) to pellitory, 146 (28.3%) to dog hair, 142 (27.5%) to cat hair, 71 (13.8%) to moulds and 102 (19.8%) to other trees. The most frequent co-sensitisation among Cs/Jc and other allergens was to pollens (grass, olive, other trees) and to cat and dog hair (Fig. 1) as compared to what was observed in the Cs/Jc non-sensitised group (p<0.001). House dust mite was the most frequent co-sensitisation allergen in this last group (p=0.003; Fig. 1).
The percentage of mono-sensitised subjects (96 – 43.24%) was lower in the Cs/Jc non-sensitised group (p<0.0001).
When we subdivided the Cs/Jc sensitised subjects into those (131–44.55%) who had or reported a worsening in symptoms during the winter (typical of Cs/Jc allergy) and those (163–55.45%) who reported symptoms during the other seasons, we found a higher number of subjects with rhinitis (89.3%) in the first sub-group in comparison with the other sub-group (79.8%) (p=0.026; see Table 2). On the contrary, in the group, which reported winter symptoms, the percentage of subjects with asthma (29%) or rhinitis+asthma (19.84%) was significantly lower if compared to the subjects with the symptoms in other seasons (asthma 52.1% – p<0.0001; rhinitis+asthma: 33.74% – p=0.008) (Table 2). A co-sensitisation to Cs and Jc allergens was found in 67.9% of the subjects with winter symptoms.
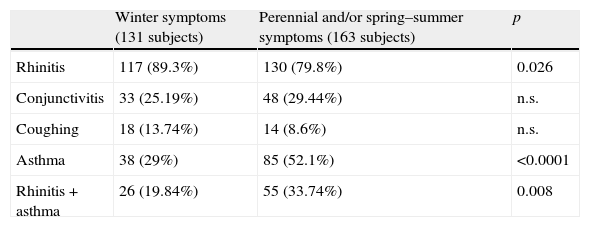
Symptoms reported by Cs/Jc sensitised subjects with winter symptoms (131) and Cs/Jc sensitised subjects with perennial and/or spring–summer symptoms (163).
| Winter symptoms (131 subjects) | Perennial and/or spring–summer symptoms (163 subjects) | p | |
| Rhinitis | 117 (89.3%) | 130 (79.8%) | 0.026 |
| Conjunctivitis | 33 (25.19%) | 48 (29.44%) | n.s. |
| Coughing | 18 (13.74%) | 14 (8.6%) | n.s. |
| Asthma | 38 (29%) | 85 (52.1%) | <0.0001 |
| Rhinitis+asthma | 26 (19.84%) | 55 (33.74%) | 0.008 |
Winter symptoms: symptoms reported from October to March; subjects that reported a worsening of symptoms in winter or had illnesses only in this season were considered.
χ2 test was applied.
The univariate analysis highlighted how all the variables tested, except for Cs/Jc sensitisation and smoking habits, were significant risk factors for rhinitis and asthma combined (Table 3). Furthermore, all allergens, except for sensitisation to moulds and smoking habits, were risk factors for rhinitis, whereas for the categories “female” and “age” (age increase) the probability of suffering from rhinitis was significantly lower (Table 3). Sensitisation to house dust mite, cat and dog hair sensitisations, age and smoking habits were risk factors for asthma (Table 3). Coughing was less frequent in subjects sensitised to grasses, mites, cat and dog hair and Cs/Jc (Table 3). Sensitisation to grasses, pellitory, olive trees, dog hair and Cs/Jc were risk factors for conjunctivitis, whereas the increase in age was a protection factor (Table 3).
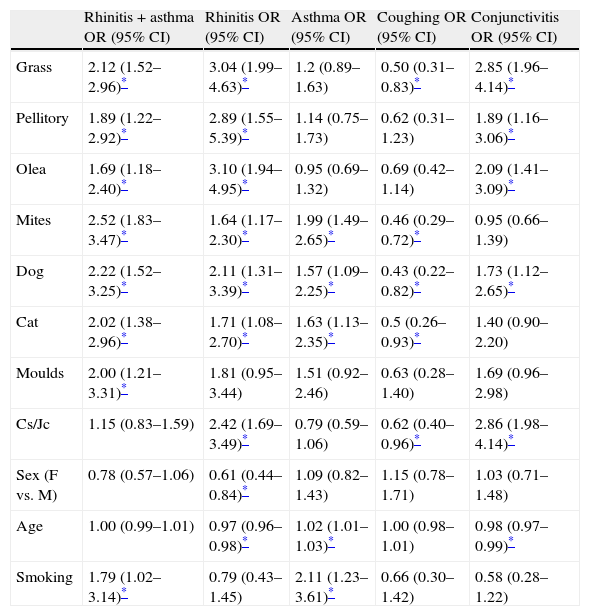
Odd ratios (OR) and 95% confidence interval (CI) calculated using an univariate logistic regression model to evaluate the relationship between allergens tested and symptoms reported.
| Rhinitis+asthma OR (95% CI) | Rhinitis OR (95% CI) | Asthma OR (95% CI) | Coughing OR (95% CI) | Conjunctivitis OR (95% CI) | |
| Grass | 2.12 (1.52–2.96)* | 3.04 (1.99–4.63)* | 1.2 (0.89–1.63) | 0.50 (0.31–0.83)* | 2.85 (1.96–4.14)* |
| Pellitory | 1.89 (1.22–2.92)* | 2.89 (1.55–5.39)* | 1.14 (0.75–1.73) | 0.62 (0.31–1.23) | 1.89 (1.16–3.06)* |
| Olea | 1.69 (1.18–2.40)* | 3.10 (1.94–4.95)* | 0.95 (0.69–1.32) | 0.69 (0.42–1.14) | 2.09 (1.41–3.09)* |
| Mites | 2.52 (1.83–3.47)* | 1.64 (1.17–2.30)* | 1.99 (1.49–2.65)* | 0.46 (0.29–0.72)* | 0.95 (0.66–1.39) |
| Dog | 2.22 (1.52–3.25)* | 2.11 (1.31–3.39)* | 1.57 (1.09–2.25)* | 0.43 (0.22–0.82)* | 1.73 (1.12–2.65)* |
| Cat | 2.02 (1.38–2.96)* | 1.71 (1.08–2.70)* | 1.63 (1.13–2.35)* | 0.5 (0.26–0.93)* | 1.40 (0.90–2.20) |
| Moulds | 2.00 (1.21–3.31)* | 1.81 (0.95–3.44) | 1.51 (0.92–2.46) | 0.63 (0.28–1.40) | 1.69 (0.96–2.98) |
| Cs/Jc | 1.15 (0.83–1.59) | 2.42 (1.69–3.49)* | 0.79 (0.59–1.06) | 0.62 (0.40–0.96)* | 2.86 (1.98–4.14)* |
| Sex (F vs. M) | 0.78 (0.57–1.06) | 0.61 (0.44–0.84)* | 1.09 (0.82–1.43) | 1.15 (0.78–1.71) | 1.03 (0.71–1.48) |
| Age | 1.00 (0.99–1.01) | 0.97 (0.96–0.98)* | 1.02 (1.01–1.03)* | 1.00 (0.98–1.01) | 0.98 (0.97–0.99)* |
| Smoking | 1.79 (1.02–3.14)* | 0.79 (0.43–1.45) | 2.11 (1.23–3.61)* | 0.66 (0.30–1.42) | 0.58 (0.28–1.22) |
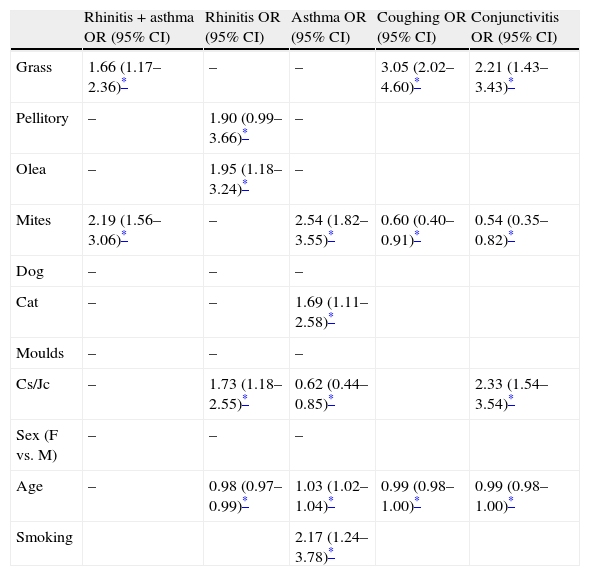
Furthermore, the multivariate analysis showed how grasses and mites were risk factors for rhinitis plus asthma (Table 4). House dust mites, as well as cat hair, were also risk factors for asthma alone, whereas, pellitory and olive were risk factors for rhinitis (Table 4). With regard to Cs/Jc, these allergens were shown to be risk factors (OR: 1.73 [IC95% 1.18–2.55]) for rhinitis, while the probability of suffering from asthma was significantly lower (OR: 0.62 [IC95% 0.44–0.85]) (Table 4). The increase in age was a protective and a risk factor for rhinitis and asthma respectively (Table 4). Sensitisation to grasses was a significant risk factor for coughing and conjunctivitis, whereas sensitisation to Cs/Jc was a risk factor only for the latter (Table 4). The probability of reporting coughing or conjunctivitis due to sensitisation to dust mites was significantly lower. The increase in age was a protective factor for these last two symptoms.
Odd ratios (OR) and 95% confidence interval (CI) calculated using a multivariate logistic regression model to evaluate the relationship between allergens tested and symptoms reported.
| Rhinitis+asthma OR (95% CI) | Rhinitis OR (95% CI) | Asthma OR (95% CI) | Coughing OR (95% CI) | Conjunctivitis OR (95% CI) | |
| Grass | 1.66 (1.17–2.36)* | – | – | 3.05 (2.02–4.60)* | 2.21 (1.43–3.43)* |
| Pellitory | – | 1.90 (0.99–3.66)* | – | ||
| Olea | – | 1.95 (1.18–3.24)* | – | ||
| Mites | 2.19 (1.56–3.06)* | – | 2.54 (1.82–3.55)* | 0.60 (0.40–0.91)* | 0.54 (0.35–0.82)* |
| Dog | – | – | – | ||
| Cat | – | – | 1.69 (1.11–2.58)* | ||
| Moulds | – | – | – | ||
| Cs/Jc | – | 1.73 (1.18–2.55)* | 0.62 (0.44–0.85)* | 2.33 (1.54–3.54)* | |
| Sex (F vs. M) | – | – | – | ||
| Age | – | 0.98 (0.97–0.99)* | 1.03 (1.02–1.04)* | 0.99 (0.98–1.00)* | 0.99 (0.98–1.00)* |
| Smoking | 2.17 (1.24–3.78)* |
On the basis of this study, the prevalence of Cs/Jc sensitisation (detected by SPTs) in outpatients with respiratory symptoms is approximately 36% in “Maremma”. Other studies had already observed that the prevalence of a positive SPT to Cypress was 28.2% in central Italy and approximately 16% in Liguria7,14 (nearby region) and 17.4% in Italy as a whole.4 The prevalence found by us would seem to be higher if compared to what was found by the studies carried out in the previous decade.4,7,12–14 This indicates how the prevalence of this allergy, which has already increased over the course of time,1,4,12–14 is still rising, both in our and other areas of Italy, where the distribution of these plants is increasing mainly for ornamental and landscape purposes. In fact, in the most recent study carried out in Apulia (southern Italy) on outpatients with respiratory symptoms, the Cupressaceae sensitisation prevalence obtained through SPT was 28.7% in the period between January and March 2003; whereas it increased to 65.5% in the same period in 2006 due to an increase in the amount of airborne pollen grains during this 3-year period (from 201g/m3 to 264g/m3 of air).8 In fact an explanation for the increasing epidemiological impact of pollinosis caused by Cupressaceae plants is related to the increasing use of these species for gardening and reforestation in Italy and in our area in the last twenty years. This is demonstrated by a progressive increase in the annual total concentrations of airborne Cupressaceae pollen in many areas of the Mediterranean1 and in particular in Italy.8,21,22 The pollen count peaks have also increased progressively over time reaching maximum daily concentration values greater than 3000–4000g/m3 of air in many Italian regions such as Tuscany, Emilia Romagna, Basilicata.23–26 In several areas of Italy the Cupressaceae represent up to 20–40% of the annual pollen count.21 Another possible explanation for this increase in the prevalence may be due to the progressive climate changes which may modify the load and the extension in duration of the pollen season and therefore influence the rate of allergic sensitisation over long periods.12,26 In fact, some authors observed an increase in the Cypress pollen counts measured in France between the 1980s and the beginning of the 21st century and in western Liguria between 1981 and 2007 with its extension in the duration of the pollen season (+18–28 days) and blooming in advance.1,27 Furthermore, in favourable weather conditions, significant pollen concentrations may be also found in October–November.2 For example, 73g/m3 of air were detected in Tuscany during the first days of December 2010.28 This prolonged presence of Cupressaceae pollens in the air in several months of the year may also be the reason for the increasing sensitisation to this allergen.
A higher number of sensitised Cs/Jc allergic subjects reported winter symptoms (typical of Cupressaceae allergy) in comparison with non-sensitised Cs/Jc subjects. However, only less than half of these subjects (44.55%) reported typical winter symptoms presumably caused by the Cs/Jc allergy. Therefore, only some Cypress sensitised patients developed illnesses related to this allergy. It is probable that in the other subjects the Cypress sensitisation was due to IgE cross-reactivity to proteins with similar structures to allergen components of other pollen and Cupressaceae extracts; IgE antibodies raised against a given allergen can bind with homologous molecules of panallergens (profiline, calcium binding protein, lipid transfer protein, thaumatin-like protein) among different botanical species.18,29 In fact, in the poly-sensitised subjects, the use of allergen extracts to detect the pollen sensitisation through SPTs can often wrongly lead to think of a co-sensitisation rather than to a cross-sensitisation due to panallergens. However, the Cupressaceae characterisation of the extract, the allergens involved and the cross-reactivity with other pollen sources still remains poorly studied. Pollen extracts from different species of the Cupressaceae family are characterised by low protein and high carbohydrate content.30 Protein and particularly carbohydrate epitopes may be involved in allergenic cross-reactivity between allergens from both taxonomically related and unrelated pollen families.16,17 In particular, olive and grass pollens contain allergens components such as Ole e 2, Phl p 12 (profilines), Phl p 7 (calcium binding protein) which may cross-react with pollens from unrelated species,18,31,32 probably also with the Cupressaceae family. Some calcium binding proteins (Cry j 4, Jun o 4, Cup a 4), profilines (Cup s 8) and thaumatins (Cup a 3, Cry j 3, Cup s 3, Jun a 3) from Cupressaceae species33 could have similar molecular characteristics to other unrelated pollen panallergens, the reason for cross-reactivity. In fact, a new allergen from C. arizonica, Cup a 4, a calcium binding protein and recently identified, has structural similarities to other calcium binding allergens such as Ole e 3, Ole e 8 and Phl p 7.34 Consequently, the Cupressaceae sensitisation may not be very important in some subjects. In fact, some authors observed that among 50 patients showing a SPT reactivity to cypress, only 37 highlighted a positive serum-specific IgE to cypress pollen and only a single mono-sensitised patient resulted positive to a nasal provocation test with C. sempervirens allergen extract.35
As confirmation to this hypothesis and similar to that found by others,35 we found a higher significant co-sensitisation among Cs/Jc and other pollen extracts, in particular to grasses (53.06%), olive (42.51%) and other trees (26.5%) unlike what was found in Cs/Jc non-sensitised patients. In fact, a higher number of Cs/Jc sensitised subjects (42.2%) reported also spring symptoms caused mainly by grass and olive. This high co-sensitisation among Cs/Jc and grass, olive and other trees may actually be a cross-reactivity among similar allergen components of these pollens, at least in some cases. However, among Cs/Jc mono-sensitised subjects the real clinical impact of this allergy was lower in our study; only 69% of them showed winter symptoms. Therefore, a positive SPT to Cupressaceae pollen extract or its reactive IgE antibodies may not reflect the true prevalence of this sensitisation.
Some authors observed that rhinitis is the most frequent symptom in Cupressaceae mono-sensitised patients (49%), followed by conjunctivitis (32%) and asthma (16%).7 In another study, the prevalence of asthma in cypress sensitised patients seemed to be very low.11 Another large French study showed that cypress allergy is characterised by rhinitis in 96.2%, conjunctivitis in 86.7%, asthma in 38% and dry cough in 16.5% of cypress sensitised subjects.1,36 A higher prevalence of dry cough and a lower prevalence of conjunctivitis was found in subjects allergic to cypress compared to patients with grass pollen allergy.1,36 In our study, rhinitis was the predominant symptom (89% vs. 79%; p<0.026), whereas asthma was less frequent (29% vs. 52.1%; p<0.0001) in Cs/Jc sensitised subjects with winter symptoms (where the influence of this allergy was most probable), compared to Cs/Jc sensitised patients who did not report any winter symptoms. According to what was found by the above-mentioned studies, the logistic regression also highlighted that Cs/Jc was a higher risk factor for rhinitis and conjunctivitis, whereas this sensitisation determines a lower probability of being asthmatic. This is probably due to the large aerodynamic size (20–30μm) of pollen grains,1 which do not reach the lower thoracic regions of the respiratory tract; they can be located in the nasal or nasopharyngeal mucous membrane12 provoking mainly rhinitis and conjunctivitis. Coughing seems to be a non-specific symptom of allergic respiratory illnesses and, in particular, of Cupressaceae sensitisation. In fact, in our study, coughing was more common in non-allergic patients. There are several non-allergic coughing causes: upper airway cough syndrome, gastro-oesophageal reflux disease, non-asthmatic eosinophilic bronchitis, upper respiratory infection, speech–language pathology and others.37
The pollen extracts of various Cupressaceae species show a high cross-reactivity and share a number of common epitopes, in particular between C. arizonica and C. sempervirens.1,38 There is also a high cross-reactivity between the major allergens of C. arizonica (Cup a 1, Cup a 3) and sempervirens (Cup s 1) and Jun a 1 and Jun a 3, the major allergens of Juniperus ashei with a homology of 75–90%.1 Furthermore, recombinant Cup a 1 is highly homologous with the major allergens of Japanese cypress (Cha o 1) and Japanese cedar (Cry j 1).39 The high degree of homology among Cup a 1 Cha o 1, Jun a 1 and Cry j 1 explains the cross-reactivity of conifer pollens.37 Also Cup s 3, an allergen of Italian cypress pollen, highlighted a cross-reactivity and homology with other pollen PR-5 proteins, such as Jun a 3.40 In our study, we found a poor co-sensitisation or cross-reactivity between Cs and Jc. In fact, only 65.9% of all Cs/Jc allergic subjects and 67.9% of those with winter symptoms showed a positive SPT sensitivity to Cs and Jc together. In the remaining 32% of subjects with winter symptoms, where the Cypress allergy was most likely, a positive SPT reactivity only to Cs was found. This supports the hypothesis that there is a reduced Jc SPT sensitivity and specificity in identifying subjects with Cupressaceae allergy compared to Cs, at least in vivo. In another study, where seven different Cupressaceae and Taxodiaceae (not J. communis) allergens were tested, C. sempervirens was the allergen with the highest sensitivity/specificity in prick tests (90%).7 Furthermore, J. ashei pollen extract demonstrated a sensitivity of 95%, a specificity of 100%, a negative predictive value of 96% and a positive predictive value of 100%.39 On the contrary, there are no epidemiological and biological studies concerning J. communis, but on the basis of our study, it is probable that the major allergen of this plant (Jun c 1)15 may not have many similarities to Cup s 1, the major allergen of C. sempervirens; alternatively, the prevalence of Jc sensitisation is lower in comparison to Cs or furthermore, the pollen extract of Jc is weaker than that of Cs. Therefore, based on what we found, the allergen extract of this species should not be used alone for the diagnosis of an allergy to Cupressaceae and consequently for a specific immunotherapy for cypress pollen hypersensitivity.
In conclusion, the prevalence of Cs/Jc sensitisation (through SPTs) in subjects with respiratory symptoms is approximately 36% in “Maremma”. However, this allergy seems to cause symptoms only in less than half of Cs/Jc sensitised subjects. Rhinitis and conjunctivitis are the predominant symptoms, whereas asthma is less frequent. The J. communis allergen is less sensitive than C. sempervirens, when testing a Cupressaceae sensitisation.
Conflict of interestAll authors declare to have no conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of the manuscript. All the authors certify that the material is original and not being considered for publication elsewhere. The authors alone are responsible for the contents and writing of the paper.
All the authors have given a significant contribution and have read and approved the submission of the manuscript.
Bruno Sposato (MD) is the principal investigator who has proposed and designed the study. He has also collected the data and written the paper. Marco Scalese (PhD) has performed the statistical analysis.