The oral allergy syndrome (OAS) is a particular type of food allergy rarely explored in the paediatric population that is already considered an adult problem.
ObjectiveIdentify the prevalence of OAS, symptoms and pollen species associated with its presence in children affected by allergic diseases.
MethodsA cross-sectional study was conducted. Consecutive sampling included children from 6 to 14 years who needed allergy treatment for the first time. A structured questionnaire was carried out to collect demographic and clinical data and history of OAS. Besides sensitisation to various allergens, the skin prick-by-prick test was performed to corroborate sensitisation to food related to OAS. Prevalence of OAS and its association with pollens was established following the covariate adjusted logistic regression.
Results267 subjects were included. Overall prevalence of OAS was 8.9% (95%CI 6.1–13.1%). Prevalence of OAS for allergic rhinitis and asthma were 8.8% and 9.1%, respectively. In patients sensitised to pollen, the prevalence ranged from 9.6% to 12.2% depending on the type of pollen. 62.5% of children with OAS were sensitive to pineapple. After adjusting for gender and family history of atopic disease, trees from the Quercus species showed an association with OAS (OR=2.7, 95%CI 1.2–6.2).
ConclusionsOAS is not uncommon in our environment. Pineapple, a typical fruit from the region, was the main food related. Quercus sp., but not birch nor olive, was the pollen associated with this syndrome.
Food allergy is the result of an immune response directed against certain antigens found in food.1 Its prevalence in children has been estimated to range from 4.8% to 6.0%.2,3
Recently, a group of patients who exhibit symptoms after consumption of food and whose skin prick tests are positive has gained notoriety.4 The oral allergy syndrome (OAS) or pollen-food syndrome (PFS) refers to a hypersensitivity reaction mediated by immunoglobulin IgE that is directed against food. Such reaction is triggered by prior sensitisation to inhaled allergens coming from plants.5 This syndrome is characterised by symptoms that are usually limited to the mouth (palate or lips itching, throbbing pain and swelling of lips, tongue, palate and throat, as well as a sensation of tightness in the throat),6 and sometimes is accompanied by extra-oral manifestations (rash, nasal or otic itching, runny nose and itchy eyes).7
OAS has rarely been explored in the paediatric population, since it is considered primarily an adult problem.8 In the USA, the prevalence in children has been estimated to be 5.0%.9 More recently, in Australia it was almost 17%,10 while in some European countries it was higher than 20%.11,12 In Latin America there are no data related to OAS in children.
Our study aimed to determine the prevalence of oral allergy syndrome within a sample of children with allergic diseases and, identify foods that are frequently associated with its symptoms and pollen species related to its occurrence.
Methods and patientsEthicsThe parents signed a written informed consent for their children to participate in the study; another one for the skin prick test; and, if necessary, one more for the skin prick-by-prick tests. The Research and Ethics Committee of the hospital approved this study.
Design and populationA cross-sectional study was conducted to analyse a sample that included 267 children older than six years old and under 15 years old who needed allergy treatment for the first time. Children with asthma, allergic rhinitis or atopic dermatitis were considered, as well as those with allergies to foods or drugs or hives. Children were recruited consecutively from July to December 2014.
Skin testingParents of the children were instructed to stop providing their children with drugs that could interfere with the interpretation of results, especially antihistamines, at least one week before performing the allergy skin tests to their children.
Allergen sensitisation was established by testing a battery of multiple glycerinated allergens common in our geographical area (house dust mites, cockroach mix, dog and cat epithelia, feathers, grass pollen, weeds and trees and fungal spores). Histamine and glycerinated solution were used as positive and negative controls respectively. The allergens were applied on the back of each child using a multiple applicator (Multitest®) and after 15min the test results were interpreted following international recommendations.13
The skin prick-by-prick test14 was performed to corroborate sensitisation to food related to OAS. The interpretation of the results also followed international recommendations.
DefinitionsFor purposes of this study, OAS was the occurrence of clinical manifestations in the oral cavity (oral pruritus, hoarseness, swelling of lips, tongue, pharynx or larynx) associated with the consumption of certain fruits, vegetables or spices15 within 30min after eating the offending food, as well as the positive skin prick test to that food. PFS was the association between OAS and sensitisation to pollens.
Statistical analysisTo determine the OAS prevalence, its frequency and respective 95% confidence intervals (95%CI) were calculated. The qualitative variables were represented as percentages, while both the mean and standard deviation were calculated for the quantitative variables. The Chi square test was used to compare qualitative variables, whereas the Fisher's exact test was used when necessary. To identify the associated pollens (independent variables) with OAS (dependent variable), odds ratios (OR) and their respective 95%CI were calculated using the multivariate logistic regression model adjusted for gender and family history of atopy. Any value of p<0.05 was considered statistically significant. The IBM SPSS software version 20.0 for Windows (Armonk, NY, USA) was used to process data.
ResultsRegarding the population analysed, 55.8% were men (149/267), while the mean age was 9.2±2.5 years (see Table 1). Over 90% of children had allergic rhinitis with or without asthma. Atopic dermatitis and food or drug allergy were less than 5%. According to the family history of atopic disease, allergic rhinitis and asthma, in that order, were the most frequent alterations in the father, mother and siblings of the children.
Description of the patients, n=267.
| Characteristic | Indicator |
|---|---|
| Age (in years), mean±SD (minimum–maximum) | 9.2±2.5 (6–14) |
| Gender, n (%) | |
| Male | 149 (55.8) |
| Female | 118 (44.2) |
| Atopic disease,an(%) | |
| Allergic rhinitis | 249 (93.3) |
| Asthma | 186 (69.7) |
| Atopic dermatitis | 7 (2.6) |
| Food allergy | 5 (1.9) |
| Drug allergy | 4 (1.5) |
| Hives | 1 (0.4) |
| Family history of atopic disease,n(%) | |
| Mother | |
| Allergic rhinitis | 75 (28.1) |
| Asthma | 51 (19.1) |
| Drug allergy | 9 (3.4) |
| Atopic dermatitis | 7 (2.6) |
| Food allergy | 4 (1.5) |
| Hives | 2 (0.7) |
| Father | |
| Allergic rhinitis | 37 (13.9) |
| Asthma | 25 (9.4) |
| Drug allergy | 4 (1.5) |
| Atopic dermatitis | 3 (1.1) |
| Food allergy | 2 (0.7) |
| Hives | 1 (0.4) |
| A sibling | |
| Allergic rhinitis | 101 (37.8) |
| Asthma | 81 (30.3) |
| Drug allergy | 2 (0.7) |
| Atopic dermatitis | 9 (3.4) |
| Food allergy | 4 (1.5) |
| Hives | 0 (0) |
SD: standard deviation.
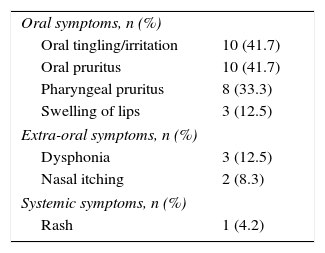
The clinical manifestations most frequently associated with OAS were as follows, oral tingling, oral pruritus and swelling of lips fewer than 15%, dysphonia was the main extra-oral symptom followed by nasal itching. One child exhibited systemic symptoms (rash) (see Table 2).
Clinical manifestations of the oral allergy syndrome in children, n=24.
| Oral symptoms, n (%) | |
| Oral tingling/irritation | 10 (41.7) |
| Oral pruritus | 10 (41.7) |
| Pharyngeal pruritus | 8 (33.3) |
| Swelling of lips | 3 (12.5) |
| Extra-oral symptoms, n (%) | |
| Dysphonia | 3 (12.5) |
| Nasal itching | 2 (8.3) |
| Systemic symptoms, n (%) | |
| Rash | 1 (4.2) |
In children with OAS, two years was the median age for the onset of oral symptoms and, the median evolution time for asthma and allergic rhinitis were three years and four years, respectively.
The prevalence of OAS was 24/267 children (8.9%; 95%CI 6.1–13.1%), while for children with allergic rhinitis was 8.8% and 9.1% for asthma. In children sensitised to any type of pollen the frequency of OAS was 9.6%, while 3.7% (p=0.485) for those sensitised to other allergens. When comparing patients sensitised to tree, weed or grass pollens with patients not sensitised to each of these pollens, the frequency of OAS was 11.3% vs. 2.8% (p=0.031), 10.3% vs. 7.4% (p=0.420) and 12.2% vs 7.7% (p=0.331), respectively. Individually, Quercus sp. (15.9% vs 5.9%, p=0.009) and Fraxinus sp. (14.9% vs 6.7%, p=0.038) were the pollens that showed a significant association with OAS. The frequency of OAS in those sensitised to pollens coming from Betulaceae, Betula sp. or Alnus sp., did not show any statistically significant association, p=0.999 and p=0.172 respectively. In sensitised patients versus non-sensitised patients to Fraxinus sp. the frequency was 14.9% vs. 6.7% (p=0.038).
The frequency of the pollen-food syndrome was 23/24 (95.8%). No latex-fruit syndrome cases were observed. Patients sensitised to Artemisia, 1/54 (1.9%), exhibited the mugwort-peach association.
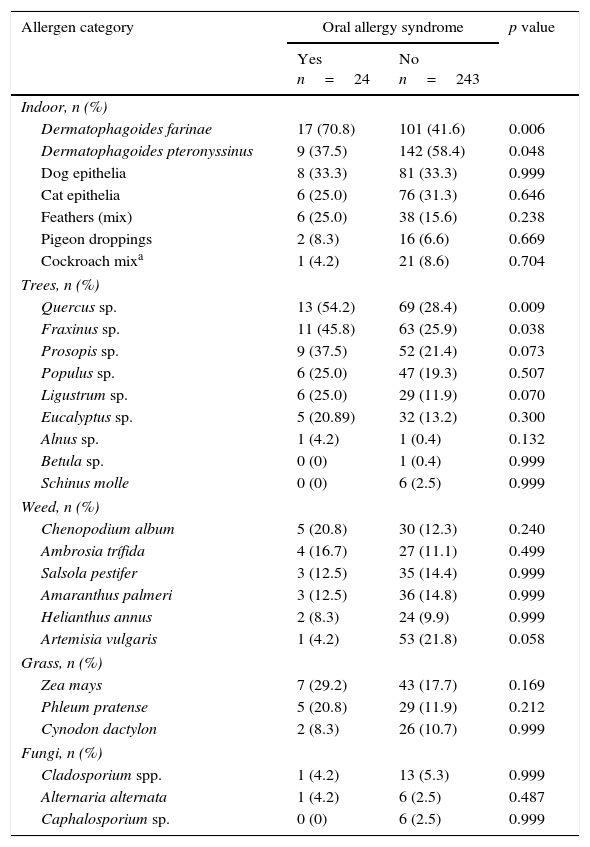
House dust mites (Dermatophagoides farinae) were significantly associated with OAS (p=0.006), (see Table 3). Regarding trees, oak (p=0.009) and ash (p=0.038), the magnitude of the association was OR 3.41 (95%CI 1.37–8.54, p=0.009), 2.98 (95%CI 1.27–6.97, p=0.012) and OR 2.42 (95%CI 1.03–5.67, p=0.042) respectively. Neither weed nor grass showed a significant association.
Frequency of sensitisation to allergens in children.
| Allergen category | Oral allergy syndrome | p value | |
|---|---|---|---|
| Yes n=24 | No n=243 | ||
| Indoor, n (%) | |||
| Dermatophagoides farinae | 17 (70.8) | 101 (41.6) | 0.006 |
| Dermatophagoides pteronyssinus | 9 (37.5) | 142 (58.4) | 0.048 |
| Dog epithelia | 8 (33.3) | 81 (33.3) | 0.999 |
| Cat epithelia | 6 (25.0) | 76 (31.3) | 0.646 |
| Feathers (mix) | 6 (25.0) | 38 (15.6) | 0.238 |
| Pigeon droppings | 2 (8.3) | 16 (6.6) | 0.669 |
| Cockroach mixa | 1 (4.2) | 21 (8.6) | 0.704 |
| Trees, n (%) | |||
| Quercus sp. | 13 (54.2) | 69 (28.4) | 0.009 |
| Fraxinus sp. | 11 (45.8) | 63 (25.9) | 0.038 |
| Prosopis sp. | 9 (37.5) | 52 (21.4) | 0.073 |
| Populus sp. | 6 (25.0) | 47 (19.3) | 0.507 |
| Ligustrum sp. | 6 (25.0) | 29 (11.9) | 0.070 |
| Eucalyptus sp. | 5 (20.89) | 32 (13.2) | 0.300 |
| Alnus sp. | 1 (4.2) | 1 (0.4) | 0.132 |
| Betula sp. | 0 (0) | 1 (0.4) | 0.999 |
| Schinus molle | 0 (0) | 6 (2.5) | 0.999 |
| Weed, n (%) | |||
| Chenopodium album | 5 (20.8) | 30 (12.3) | 0.240 |
| Ambrosia trífida | 4 (16.7) | 27 (11.1) | 0.499 |
| Salsola pestifer | 3 (12.5) | 35 (14.4) | 0.999 |
| Amaranthus palmeri | 3 (12.5) | 36 (14.8) | 0.999 |
| Helianthus annus | 2 (8.3) | 24 (9.9) | 0.999 |
| Artemisia vulgaris | 1 (4.2) | 53 (21.8) | 0.058 |
| Grass, n (%) | |||
| Zea mays | 7 (29.2) | 43 (17.7) | 0.169 |
| Phleum pratense | 5 (20.8) | 29 (11.9) | 0.212 |
| Cynodon dactylon | 2 (8.3) | 26 (10.7) | 0.999 |
| Fungi, n (%) | |||
| Cladosporium spp. | 1 (4.2) | 13 (5.3) | 0.999 |
| Alternaria alternata | 1 (4.2) | 6 (2.5) | 0.487 |
| Caphalosporium sp. | 0 (0) | 6 (2.5) | 0.999 |
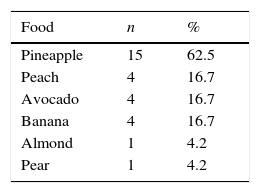
The skin prick-by-prick test showed that pineapple (62.5%) followed by peach, avocado and banana (16.7% each) were the foods mainly involved in OAS (see Table 4).
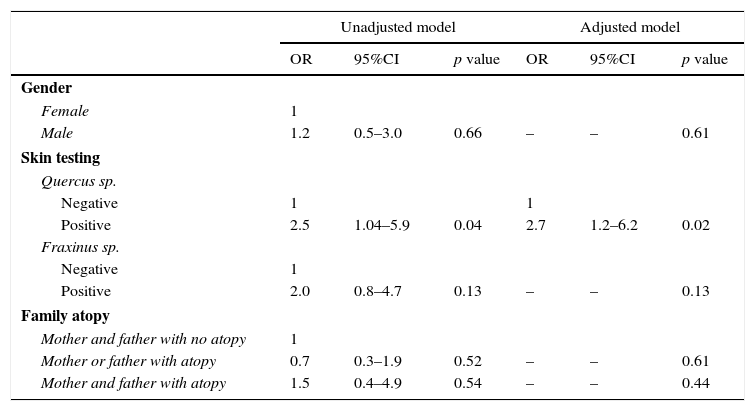
The multivariate analysis identified the Quercus species as the main factor associated with OAS (see Table 5). Factors such as family history of atopic disease or gender were excluded from the final model because they did not show any significant association.
Association between pollen and oral allergy syndrome adjusted for gender and family atopy.
| Unadjusted model | Adjusted model | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p value | OR | 95%CI | p value | |
| Gender | ||||||
| Female | 1 | |||||
| Male | 1.2 | 0.5–3.0 | 0.66 | – | – | 0.61 |
| Skin testing | ||||||
| Quercus sp. | ||||||
| Negative | 1 | 1 | ||||
| Positive | 2.5 | 1.04–5.9 | 0.04 | 2.7 | 1.2–6.2 | 0.02 |
| Fraxinus sp. | ||||||
| Negative | 1 | |||||
| Positive | 2.0 | 0.8–4.7 | 0.13 | – | – | 0.13 |
| Family atopy | ||||||
| Mother and father with no atopy | 1 | |||||
| Mother or father with atopy | 0.7 | 0.3–1.9 | 0.52 | – | – | 0.61 |
| Mother and father with atopy | 1.5 | 0.4–4.9 | 0.54 | – | – | 0.44 |
Model adjusted following the Forward conditional method.
OR obtained following logistic regression.
To the best of our knowledge this is the first study in Mexico to document that 10% of children with allergic diseases develop OAS. Therefore, it is shown that OAS is not uncommon in Mexican schoolchildren and fruits, in this case pineapple, were the foods most often associated with its symptoms. Finally, we were able to identify the Quercus species as a factor strongly associated with OAS.
The occurrence of OAS in the paediatric population has rarely been explored. In our study the frequency in children with allergic diseases was about 9%. To date few studies have analysed in detail the prevalence of OAS in children. In the USA a survey among allergists reported a frequency of 5% (range, 0–75%) in children with some type of allergic disease.9 In Australia, the prevalence was 14.7%.10 Recently, in a sample of 1360 Italian children with pollen-induced allergic rhinitis the prevalence of OAS was 24%.11 Similarly, Croatian children with seasonal allergic rhinitis developed OAS in 26.7% of cases.12 At present no studies on children have been carried out in our country before, hence, we are temporarily unable to know the true extent of the problem. Therefore, our results have become the starting point for further studies involving different geographic areas from our country.
According to our results, the prevalence of OAS in children differed from that observed in the adult population from our country, where it ranged from 13% to 50%7,16 or that reported in other regions of the world.8,17–19 This may be explained because some children have difficulty expressing their symptoms. Thus, in our study we decided to analyse children aged over or equal to six years to overcome this communication barrier. Another reason could be the evolution time of the allergic disease, particularly allergic rhinitis. On the other hand, it has been proposed that mothers of children tend to say their children do not like or do not want to eat some foods, when in fact they are allergic to those foods.12 Thus, in these cases it is not possible to corroborate sensitisation to food related to OAS. Finally, the geographical location is an important factor to consider. Recently, it was shown that children living in northern Italy have a higher prevalence of OAS than those who live in the south and this difference is dependent on the type and concentration of vegetation, especially birch.11
In general, patients with allergic rhinitis, particularly those sensitised to pollens, show a greater tendency to develop OAS. In our patients the frequency of OAS associated with allergic rhinitis or asthma was not higher than 10%. Also, a similar percentage was observed in children sensitised to pollens. In our country the frequency of OAS in adults sensitised to Oleaceae sp. pollens, including Fraxinus, was higher than 50%.16 This result differs notably with that observed in our study, where the frequency of OAS associated with ash trees was 15%.
Several studies in Europe have shown that birch pollen is a key player strongly linked to OAS.20,21 However, our city is not aware of the existence of this type of tree in the forest areas that surround it. Instead, Alnus species, which fall into the same category as birch (Betulaceae), have been discovered in the mountain ranges of our state, more than 100km away and over 2000m above sea level. Consequently, both factors could help explain why the low frequency of sensitisation to birch and alder pollen observed in our children (less than 1% and 5% respectively). Thus, the prevalence of OAS is expected to be lower compared to other regions of the world.
Quercus sp. and Fraxinus sp. showed an association with OAS in our study, both species are very common in our region. The first are located in an ecological reserve called Bosque de la Primavera (Spring Forest) and the latter in the city itself. However, in the multivariate analysis the Quercus species remained the only factor associated with OAS. Although oak and ash showed high rates of sensitisation in children, the frequency of OAS was nearly 15%. A possible explanation may be the low consumption of foods associated with these trees, such as the fruits from the Rosaceae species.
Interestingly, we observed an association between OAS and sensitisation to house dust mites. In our view, we have no information to explain this association, so we believe that it could be a chance finding.
Compared with other studies, in our study the fruit most often related to OAS was the pineapple (Ananas comosus). In Australian children, watermelon was the fruit most related (6/24), while pineapple only appeared in three cases.10 The kiwi, followed by peach, was the fruit most often related to OAS in a group of children with pollen-induced allergic rhinitis in Italy; no case involving pineapple11 was documented. The pineapple is a tropical fruit usually eaten raw or as juice, circumstances that favour allergens related to OAS to remain unaltered by processing or cooking. Different allergens from pineapple have been characterised, where Ana C1 and Ana C2 are the main ones. The first is a profilin and the latter a protease. An explanation of why our children showed a greater sensitisation to pineapple can be found in the sensitisation to ashes and oak, since profilins (Fra e 2 and Que a 2) have been identified in these trees. These profilins can cross-react with pineapple profilin (Ana C1).22 Another explanation may be the availability of pineapple, as the semi-tropical climate of our region favours the cultivation of this fruit so that its consumption is facilitated.
Several studies on adults have shown that the sensitisation process to pollens occurs prior to the onset of symptoms of OAS.7,23,24 However, this behaviour in children has rarely been explored. In our study we observed that allergic rhinitis or asthma occurred prior to the beginning of OAS. As expected, all children with OAS displayed some sort of symptoms in the mouth. However, it should be noted that the occurrence of systemic symptoms (4.2%) differed from that observed in adults, where it ranges from 15% to 20%.7,9 It is very likely that the evolution time of the allergic disease is related to the progression of OAS symptoms. The lack of evidence of oral challenges with offending food in our children did not allow us to specify the real frequency of systemic symptoms.
Nowadays, there is an ongoing dispute about the best term to describe the group of people with oral symptoms associated with food consumption. It seems that the pollen-food syndrome concept is gaining greater acceptance.4–6,9 In our study most of the children with oral symptoms from food (23/24) were sensitised to some kind of pollen. Additionally, most of the foods involved were of plant origin. Thus, this would be the most appropriate term to describe, at least in children, this population.
This study has some limitations. The prevalence of OAS was calculated in children with allergic diseases, so this could represent an overestimation of the real prevalence in the paediatric population. On the other hand, the lack of an oral challenge with offending food did not allow us to obtain objective data about the frequency and severity of oral symptoms. Finally, the cross-reaction between sensitisation to pollens and foods of plant origin could not be specified.
In conclusion, the prevalence of OAS in children with allergic diseases is common. Tropical fruits, including pineapple, are the main food-related oral symptoms. Tree species other than birch or olive were strongly associated with OAS.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
FundingNone.
Conflicts of interestThe authors have no funding or conflicts of interest to disclose.