Allergic rhinitis affects 10–30% of children in developed countries and has increased in frequency over the last few decades, probably due to changes in the environment and life style.
AimTo assess the prevalence, severity, and factors linked to rhinitis in 10 and 11-year-old children from Almeria (Spain).
MethodsAs part of ISAAC II, a cross-sectional survey was conducted among a representative sample of 1143 schoolchildren in spring and autumn of 2001, using homologated questionnaires and skin-prick testing.
ResultsThe overall prevalence of rhinitis and rhinoconjunctivitis were 38.9% and 24.8%, respectively, 17.9% had medically diagnosed rhinitis. During the previous year symptoms disturbed daily activities and school attendance in some measure in 40% and 26% of children with rhinitis, respectively.
The risk factors found in the multiple logistic regression analysis were atopy (OR 2.57; 95% CI 1.92–3.42); cat contact at home during first year of life (OR 2.4 95% CI 1.13–5.12); prior medical diagnosis of asthma (OR 2.2; 95% CI 1.22–4.02); nocturnal cough in absence of colds (OR 1.9; 95% CI 1.25–2.97); diagnosis of rhinitis in one of the parents (OR 1.8; 95% CI 1.31–2.59); wheezing at any time (OR 1.6; 95% CI 1.18–2.28); and nursery school attendance (OR 1.5; 95% CI 1.21–2.5).
ConclusionsThe prevalence of rhinitis found is superior to that of other centres participating in the ISAAC Phases I and II, and coexists with asthma and eczema in many children. The independent risk factors associated to rhinitis are in accordance with previous reports.
During the last 30–40 years there has been an increase in childhood allergic diseases and sensitisation1 to such an extent that the phenomenon has been called an “allergic epidemic”. Since important variations in population genetics cannot have taken place in such a short period of time, the underlying cause may correspond to changes in environmental factors.2
To be exact, rhinitis is the most frequent allergic disorder among the paediatric population, with figures above 25% in developed countries. Moreover, it produces troublesome symptoms that affect school performance and socialization,3,4 and should not be regarded as a minor disorder but as a chronic disease that has important personal, social and economic consequences.5 There are important differences in the prevalence of rhinitis, both within a single country and between different countries, due to environmental, alimentary and climatic factors among others. Variations in diagnostic criteria and study methodology may also influence these differences.
The ISAAC project (International Study of Asthma and Allergies in Childhood) was created in 1991 with the aim of establishing and comparing the prevalence of asthma and other allergic disorders in each country, using a standardised methodology. To this effect, the study used a questionnaire comprising simple questions. The present study is part of Isaac Phase II, designed to investigate allergic disorder associated factors observed in Phase I.
In this study, we aim to assess the prevalence, severity, and factors associated to allergic rhinitis in 10–11-year-old children in the city of Almeria (South-East Spain).
Population and methodsIn the spring and autumn of 2001 a cross-sectional study was made to analyse the relationships between independent variables (environmental conditions, personal and familial factors) and the frequency of allergic rhinitis. The ISAAC II methodology has been described in detail previously6; in that phase the parents or tutors completed homologated questionnaires on respiratory, nasal and skin symptoms manifesting in the previous year and at other times in the past. Questions were also included relating to personal and family antecedents, living habits, the domestic environment and the use of treatments and healthcare services. Ethical approval of the study was obtained from the Clinical Research Ethics Committee of Torrecárdenas Hospital, as well as from the Regional Health and Education Authority. The study population consisted of 10 and 11-year-old schoolchildren from 29 state schools in the city of Almeria, randomised according to the option B of the ISAAC II sampling methods,6 with a final participation of 1143/2293 children.
For the present study, atopy was taken to be synonymous of allergic sensitisation, the latter being understood as representing positivity to at least one allergen, as determined by skin prick testing. A prick test was regarded as positive by a wheal 3mm or greater than negative control. The battery of test allergens included the following extracts (ALK-Abelló®, Madrid, Spain): Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat dander, Alternaria, grass pollen mixture (Dactylis glomerata, Lolium perenne, Festuca pratensis, Poa pratensis, Phleum pratense and Avena eliator), tree pollen mixture (Betula verrucosa, Alnus glutinosa and Corylus avellana), positive control (histamine) and negative control (saline solution).
The SPSS® version 12.0 statistical package for Microsoft Windows® was used for data analysis. Calculations were made for the prevalence of symptoms and the frequency of the rest of the variables based on the ratio between the number of positive responses and the number of questionnaires completed for each question. The chi-squared test (χ2) was used to evaluate the association between the dependent variable (rhinitis) and dichotomic variables, while logistic regression analysis was used to assess the association to polychotomic variables. Statistical significance of associations was accepted for p<0.05. Unconditional multiple logistic regression analysis was made with all the variables yielding p≤0.20, defining (estimating) a model of parameters that are independently associated to rhinitis.
Definition of rhinitisThe current presence of rhinitis was defined by a positive response to the question: “Has your son/daughter sneezed, has he/she had a runny or blocked nose without having a cold or influenza during the last 12 months?” The occurrence of rhinitis in the past was confirmed by a positive answer to the question: “Has your son/daughter ever sneezed, had a runny or blocked nose, without having a cold or influenza?”
Current rhinoconjunctivitis and medical diagnosis of rhinitis were defined by affirmative answers to the questions: “Has your son/daughter had these nasal problems accompanied by itchy or watering eyes during the last 12 months?” and “Has your son/daughter ever had allergic rhinitis?” respectively (the last being a traslation from the original ‘hay fever’ in the original English version).
ResultsA total of 1143 schoolchildren participated in the study (49.8% participation rate) with a mean and median age of 10.7 years (95% of the sample aged between 9.50 and 11.85 years). There was a slight male predominance (52%) and 98.7% were of Spanish nationality.
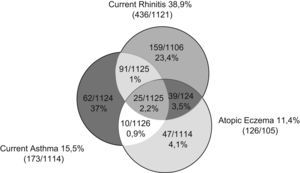
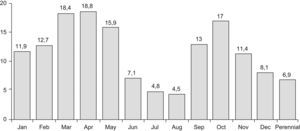
Figure 1 shows the interrelated prevalence of allergic disease symptoms in the children in the study: 45.9% show one or more symptoms of asthma, rhinitis and/or atopic eczema, while 54.1% suffered none of the three disorders. Prevalence of rhinitis during the previous year was 38.9% (436/1121), concomitant with asthma in 8.1%, with atopic eczema in 3.5% and with both disorders in 2.2% of the children. Rhinoconjunctivitis frequency was 24.8% and a medical diagnosis of rhinitis existed in 17.9% of the sample (Table 1). Seasonal incidence had two peaks, one in spring and one in October, with 6.9% of the children presenting perennial rhinitis (Figure 2). Rhinitis during the previous year was considered severe when the symptoms perturbed normal daily activities or school attendance, which occurred at least once in 175/436 (40%) and 114/436 (26%) respectively (Table 1).
Symptoms, frequency and intensity of current and past rhinitis
| n | Freq. | % | |
| Sneezing, rhinorrea or blocked nose at any time (in absence of colds or flu) | 1129 | 501 | 44.4 |
| Rhinitis during previous year | 1121 | 436 | 38.9 |
| Rhinoconjuntivitis during previous year | 1103 | 273 | 24.8 |
| Has your child ever had allergic rhinitis? | 1096 | 196 | 17.9 |
| Rhinitis has interfered with daily activities | 436 | ||
| +Never | 261 | 59.9 | |
| +Occasionally | 148 | 33.9 | |
| +Often | 24 | 5.5 | |
| +Many times | 3 | 0.7 | |
| School days lost during last year due to rhinitis | 436 | ||
| None | 322 | 73.8 | |
| 1–5 | 92 | 21.1 | |
| 6–10 | 16 | 3.6 | |
| >10 | 6 | 1.3 | |
The prevalence of allergic sensitisation was 42.6%. The distribution of positive allergens was as follows: Dermatophagoides pteronyssinus (36.2%), Dermatophagoides farinae (32.3%), cat dander (10.8%), alternaria (7%), grass pollen (6%), and tree pollen (1.7%).
We found no significant association between rhinitis and gender, weight, or type of feeding at birth. There is, however, a significant association between rhinitis and atopy (OR 3.12; CI 95% 2.4–4.03; p<0.001); wheezing in the past (OR 3.14 CI 95% 2.44–4.04; p<0.001); in the present (OR 4.5; IC 95% 3.1–6.4; p<0.001); and during or after exercise (OR 4; CI 95% 2.6–6.3, p<0.001). There is a greater risk of rhinitis in the case of wheezing unrelated to colds (OR 6.9; CI 95% 3.8–12.7; p<0.001); nocturnal cough in absence of colds (OR 5.06; CI 3.6–6.9; p<0.001); medical diagnosis of asthma (OR 5.07; CI 3.5–7.3; p<0.001); and also with the severity of the asthma, understood as wheezing severe enough to make the child's speech difficult (OR 6.6; CI 95% 2.7–16.4; p<0.001); having suffered more than 4 crises in the previous year (OR 8.8; CI 95% 3.6–21.7; p<0.001); and nocturnal awakening due to asthma (OR 6.3–7.9 according to the frequency of sleep alterations). There is a significant association with atopic eczema (OR 1.98; CI 95% 1.36–2.88; p<0.001), with increased risk if the eczemas cause nocturnal awakening (OR 3.5–6.2 depending on the number of days with disturbed sleep).
Schoolchildren in Almeria have adequate vaccination compliance (86–90%). We have not found an association between rhinitis and the state of vaccination against measles, tuberculosis or whooping cough. An association exists in children who suffered intestinal parasitation (OR 1.5; IC 95% 1.14–1.98; p=0.003) and, although with low statistical significance, whooping cough (OR 1.8; IC 95% 1.004–3.25; p=0.046).
A history of some allergic disorder was attributable to 29.1% of mothers and 20.2% of fathers. This predominance in the mothers is repeated for asthma (9.3% vs 5.8%), rhinitis (14.9% vs 10%) and atopic eczema (16.7% vs 10.6%). Among the parental variables we found the following risk factors for rhinitis: any allergic disorder in the mother (OR 1.68; CI 95% 1.3–2.2; p<0.001), in the father (OR 1.46; CI 95% 1.08–1.95; p=0.012) or either parent (OR 1.7; CI 95% 1.32–2.16; p<0.001), more especially a mother with allergic rhinitis (OR 1.99; CI 95% 1.42–2.77; p<0.001) or atopic eczema (OR 1.48; CI 95% 1.07–2.048; p=0.015) and a parent with allergic rhinitis (OR 2.13; CI 95% 1.44–3.16; p<0.001).
Seventy-seven percent of the children attended nursery school (median age of commencing: 2 years). Older siblings are a protective factor associated with rhinitis (OR 0.75; CI 95% 0.59–0.96; p=0.023), while nursery school attendance constitutes a risk factor (OR 1.57; CI 95% 1.16–2.11; p=0.003). There were birds and dogs respectively in 14.2% and 10% of the homes during the first year of life (26.2% and 22.9% during the year prior to the survey). A higher risk of rhinitis was found in children who had contact with cats during the first year of life (OR 2.48; CI 95% 1.27–4.8; p=0.006).
At the time of response, 69.4% of the families lived in urban or suburban areas (73.3% during the first year of life). In the great majority of homes there was no floor covering, there were single-glazed windows, gas was the most commonly used cooking fuel and electricity was used for heating. In addition, 5% of the homes presented damp spots, and 1.5% had visible fungal presence (10.5% and 4.5% respectively during the first year of life of the children). In 6 out of 10 households at least one person smoked and 46.6% of the mothers smoked. The majority of children used blankets and bedclothes of synthetic material. We did not find any statistically significant association between rhinitis and the location of the home or exposure to tobacco smoke (prenatal, first year of life and current), the type of fuel used for cooking or heating, the type of window and the floor covering. On the other hand, damp (OR 1.87; CI 95% 1.09–3.22; p=0.02), visible fungi presence in the house (2.90; CI 95% 1.06–7.92; p<0.029) and synthetic pillows (OR 1.35; CI 95% 1.05–1.73; p=0.016) are considered risk factors. A feather pillow, however, is a protective factor (OR 0.46 CI 95% 0.23–0.92; p=0.024). No significant association was found between rhinitis and other bedding and pillow materials.
The children eat fruit and fresh and cooked vegetables nearly every day: 67%, 51.9% and 22.5% respectively; fish 28.2% and meat 62.8%. 33.7% consume hamburgers, 55.5% fizzy drinks and 62.3% factory baked goods more than once a week. 77.3% of the children do physical exercise outside school at least 2 days a week, although the survey does not specify the characteristics (duration, intensity) of the physical activity carried out. We did not find any association between eating habits or practice of sports and rhinitis.
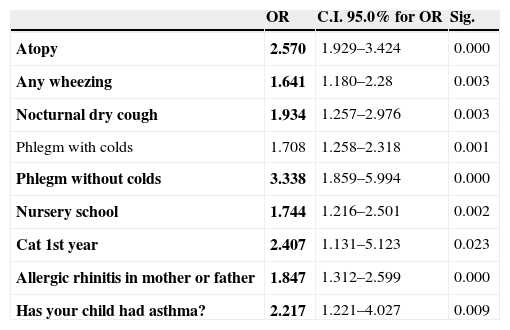
Following the multiple logistic regression analysis made for the children in the sample, with the other variables analysed, the factors found to be linked to rhinitis were: presence of atopy (OR 2.57; 95% CI 1.92–3.42; p<0.001), prior medical diagnosis of asthma (OR 2.2; 95% CI 1.22–4.02; p=0.009), nocturnal cough in absence of colds (OR 1.9; 95% CI 1.25–2.97; p=0.003), wheezing at any time (OR 1.6; 95% CI 1.18–2.28; p=0.003), nursery school attendance (OR 1.5; 95% CI 1.21–2.5; p=0.002), diagnosis of parental allergic rhinitis (OR 1.8; 95% CI 1.31–2.59; p<0.001), and contact with cats during the first year of life (OR 2.4 95% CI 1.13–5.12; p=0.023). The variables phlegm with or without cold were not taken into account because they are symptoms of rhinitis (Table 2).
Factors linked to rhinitis assessed by multivariate logistic regression
| OR | C.I. 95.0% for OR | Sig. | |
| Atopy | 2.570 | 1.929–3.424 | 0.000 |
| Any wheezing | 1.641 | 1.180–2.28 | 0.003 |
| Nocturnal dry cough | 1.934 | 1.257–2.976 | 0.003 |
| Phlegm with colds | 1.708 | 1.258–2.318 | 0.001 |
| Phlegm without colds | 3.338 | 1.859–5.994 | 0.000 |
| Nursery school | 1.744 | 1.216–2.501 | 0.002 |
| Cat 1st year | 2.407 | 1.131–5.123 | 0.023 |
| Allergic rhinitis in mother or father | 1.847 | 1.312–2.599 | 0.000 |
| Has your child had asthma? | 2.217 | 1.221–4.027 | 0.009 |
OR: odds ratio; CI: confidence interval; Sig: statistical significance.
Most studies on the prevalence of rhinitis are based on the number of patients requesting medical care and, therefore, undervalue the real frequency of the disease because patients who do not consult a doctor specifically for this reason are excluded. On the other hand, population studies probably reflect the real situation better, although such surveys could show overdiagnosis due to a selection bias.
The overall participation rate was 49.8% (1143/2293). The main reasons for non-participation were doubts about the aim of the study, rejection to skin prick or blood test or questionnaire cumplimentation (90%), children out of age range (8%); and refusal of the pupils (2%). Surveys conducted in a school framework run the risk of participation bias, being more prone to sign a consent those parents with family or personal history of allergic diseases, justifying a larger prevalence of atopy and atopic diseases. Another factor affecting selection bias is that repeated use of the schools to perform socio-sanitary programs (Almeria had already participated in ISAAC I) could predispose the parents to a non-collaborative attitude. However, the response rate was similar to the other three Spanish centres participating in ISAAC II: Cartagena (58.9%), Valencia (43.7%), and Madrid (53.2%),7 and higher than another survey which did not show differences in some allergic diagnoses between responders and non-responder pupils.8
In our study the prevalence of rhinitis (38.9%) was lower to that found (44.3%) in 13–14 year-old adolescents in Cantabria (Spain)9 and higher to that observed by the Spanish Group of ISAAC I (31.3%) in the same age group.10 Likewise, it is superior to that found in other groups in ISAAC Phase II in Rome11 (13.2%) and Ankara12 (30.6%) in 10–11 year-old children. The reduced prevalence of medical diagnosis of rhinitis (17.9%), compared to the self-assessment made by parents, could indicate an infra-diagnosis since patients do not seek medical care for less severe symptoms. However, the prevalence of rhinoconjunctivitis during the previous year was 24.8%, a figure greater to those observed in adolescents by both Asher et al.,13 and the Spanish Group of ISAAC I (19% and 15.4%, respectively).10 In Spain, a prior publication observed geographical variations in the prevalence of rhinitis, suggesting that local differences in risk factors could be involved.14
In coincidence with other investigators,15,16 we found that gender did not have significant influence on the presence of rhinitis although it did affect atopic sensitisation (1.64 times greater in males). Moreover, we have not found that birth-weight nor type and duration of infant feeding are associated with rhinitis. It has been suggested that breastfeeding prevents allergic disorders in children without parental allergic antecedents, but not in the subgroup with a family history of atopy,17 which could lead to confusion by introducing reverse causality as such children have an important burden of atopy and their families voluntarily apply measures such as prolonging breastfeeding and avoiding pets and tobacco smoke in the home. Two meta-analyses published in 2001 conclude that breastfeeding during at least 4 months offers protection against asthma and atopic eczema in infancy,18,19 the effect being more pronounced in children with a family history of atopy, but conclusions about rhinitis were not accomplished.
In our study, atopy multiplies the risk of rhinitis by three. Moreover, rhinitis is associated to the presence of asthma and atopic eczema (Figure 1), which confirms the relation between the distinct allergic disorders mediated by IgE, influenced by common genetic and environmental factors. The increased prevalence of asthma (9.3% vs 5.8%), rhinitis (14.9% vs 10%), and atopic eczema (16.7% vs 10.6%) in the mothers compared with fathers could constitute a bias due to the fact that it is generally the mothers who complete the questionnaires (81.3%). They may give more consideration to their own symptoms and undervalue those of the fathers, who could show lower perception and communication of symptoms.20 In addition, there is a possible hormonal influence on allergic diseases.21 The presence of rhinitis or eczema in the mother and of rhinitis in the father increased the risk of rhinitis in children. These data agree with the classic study of Kjellman22 on family grouping of allergic diseases.
We found no relation between rhinitis and vaccination against measles, tuberculosis or whooping cough, subject of an ample debate over recent years with data in favour of23 and against24,25 the association between vaccination and allergic disorders. Intestinal worms and whooping cough were found to increase the risk of rhinitis, the latter with low statistical significance. Similarly to the findings obtained in a study carried out in Ecuador,26 we observe that parasitic infections are associated with a lower incidence of sensitisation (data not shown), but this does not result in a lower incidence of allergic disorders.
Our results related to exposure to other children are contradictory, but in consonance with others.27 On the one hand, we find that the presence of older siblings constitutes a protective factor, while nursery school attendance is a risk factor. Most published studies associate exposure to other children with lower allergic disease rates. However, when evaluating these studies, other confounding factors must be taken into account, such as the number and ages of people living in the home, socioeconomic status, family antecedents of atopy or infections suffered during infancy.
We found that schoolchildren exposed to cats during the first year of life had a higher risk of rhinitis. Controversy exists regarding the possession of domestic pets: it has been suggested that this may constitute a protective factor,28,29 although there are publications that consider it to be a risk factor.30,31 This fact may reflect a reversal bias, and could depend on the type of pet and the time and duration of the exposure, or may vary among subgroups (with or without familial or personal atopy).8
We have not found an association between passive smoking and rhinitis. Although the relation between exposure to tobacco smoke (present and past) and asthma is evident,32 association with atopy and other allergic disorders is not clear.33,34 We have not observed an association between rhinitis and exterior insulation or floor covering in the bedroom. Current exposure —although not in the first year of life, in disagreement with Kuyucu et al.12— to damp patches and/or visible fungi in the home increases the risk of rhinitis. Although damp itself is not a cause of rhinitis, it could be connected with sensitisation to dust mites and or Alternaria (allergens that proliferate in damp environments) acquired over time.
We have not found a relation between the different types of fuel used for cooking and heating and the presence of rhinitis. The different fuels used for cooking and heating have been considered risk factors for childhood respiratory symptoms,35 but their effect on rhinitis has not been studied extensively. Zacharasiewicz et al.36 find synthetic bedding, presence of damp and/or fungi and gas central heating to be risk factors for rhinitis. In our study the use of a synthetic pillow in the previous year is a risk factor for rhinitis, while a feather pillow is a protective factor. Other studies have found a positive association between wheezing and rhinitis in the previous year and synthetic bedding,36,37 since it contributes to the proliferation of dust mites, while the use of feather eiderdowns is associated with a lower risk of wheezing and dust mite sensitisation.38
The children's diet does not meet the standards of the Mediterranean diet and is closer to Anglo-Saxon habits, although we have not found an association between eating habits or the practice of sports with rhinitis. There are on-going studies on the relation between consumption of anti-oxidants, vitamin D and monosaturated fats and allergic diseases.39,40
One of the limitations of the study is the low-response rate, contributing to a selection bias by which families with atopic antecedents are more willing to enroll on the survey and to complete the questionnaires more properly, leading to an overestimation of the prevalence of rhinitis. Another important concern is that the associated factors could be of a not exactly causative nature, this being attributable to the cross-sectional design. Nevertheless, the strength of this study is to be framed within ISAAC phase II, and the observed associations are in agreement with most of the related studies.
ConclusionsThere is a high prevalence of rhinitis in 10–11 year-old schoolchildren in the city of Almeria, superior to that found in other centres participating in ISAAC Phase II. Rhinitis coexists with asthma and atopic eczema in a high percentage of children, causing important limitations in their daily activities and a greater use of healthcare resources.
The rhinitis risk factors found in our study are: personal history of atopy and asthma; parental allergic rhinitis; contact with cats during the first year of life; and nursery care attendance. These data coincide with other studies published on rhinitis in children.
Conflict of interestNone of the authors declare conflict of interest in relation to this work.