The objectives of this study were to determine the prevalence of asthma and allergies in 13- to 14-year-old adolescents in the city of Taubaté, São Paulo, Brazil using the INTERNATIONAL STUDY OF ASTHMA AND ALLERGIES IN CHILDHOOD (ISAAC) questionnaire and to describe the presence of risk factors in current asthma carriers.
MethodsWe used a cross-sectional study involving 920 adolescents who completed the ISAAC questionnaire and answered additional questions regarding smoking and the presence of pets and/or insects at home.
ResultsThe mean prevalence rate of “current asthma” was 15.3% and “asthma ever” was 6.8%. The mean prevalence rate of “current rhinitis” was 36.6% and “rhinitis ever” 37.6%. The prevalence of “eczema ever” was 16.2%. The frequency of active smoking was low (0.7%), and the presence of indoor animals (34%) and of insects (55.1%) was high.
ConclusionsThe prevalence of “current asthma” was twice as high as that of “asthma ever”. There was no association between risk factors studied and current asthma.
Allergic asthma, rhinitis, and atopic eczema are among the commonest causes of chronic ill health in the world. Asthma is one of the most common chronic conditions affecting both children and adults, yet much remains to be learned of its aetiology. Although genetic predisposition is clearly evident, gene-by-environment interaction probably explains much of the international variation in prevalence rates for allergy and asthma. Environmental factors such as infections and exposure to endotoxins may be protective or may act as risk factors, depending in part on the timing of exposure in infancy and childhood. Some prenatal risk factors, including maternal smoking, have been firmly established, but diet and nutrition, stress, use of antibiotics and mode of delivery may also affect the early development of allergy and asthma. Later in childhood, putative risk factors include exposure to allergens, breastfeeding (which may initially protect and then increase the risk of sensitisation), family size and structure, and sex and gender.1
Allergic rhinitis is a common disease characterised by nasal itch, sneezing, watery and mucous rhinorrhoea, and nasal obstruction.2
Atopic dermatitis (AD) is a common chronic relapsing inflammatory skin disease, characterised by intense itching, dry skin, inflammation and exudation.3
Asthma is a public health problem in several countries, and the study of its prevalence and the risk factors associated with the disease is of global interest.4–7 In Brazil, there are 350,000 asthma-related hospitalisations per year, constituting the fourth leading cause of hospitalisation by the Unified Health System (2.3% of the total) and the third leading cause of hospitalisation among children and young adults.8 However, until the last decade, the prevalence of asthma and allergies could not be properly assessed due to the lack of a standardised methodology that allows for comparative studies. The need for a globally standardised method was the first step toward the creation of the INTERNATIONAL STUDY OF ASTHMA AND ALLERGIES IN CHILDHOOD (ISAAC). Results from the ISAAC PHASE I showed differences in the prevalence of asthma in the 56 countries studied. The prevalence of current asthma (wheezing in the last 12 months) in the population of children 6-7 years of age ranged from 0.8% to 32.1%. Among adolescents between the ages of 13 and 14 years, the prevalence of current asthma ranged from 2.1–4.4% in Albania, China, Greece, Georgia, Indonesia, Romania and Russia, to 29.1–32.2% in Australia, New Zealand, Republic of Ireland and the UK. The two centres at the extremes of prevalence were Akola (1.6%) and Scotland (36.7%).4
In Brazil, seven centres (Recife, Salvador, Uberlândia, Itabira, São Paulo-Sul, Curitiba and Porto Alegre) participated on the first phase of ISAAC and the results were similar to the other centres. On the third phase of ISAAC 21 centres in Brazil (with 58,144 questionnaires answered by adolescents) participated with variable results for asthma, rhinitis and eczema between centres, but with higher prevalence rates at centres closer to the equator. The mean prevalence rates were 19% for current asthma, 14.6% for rhino-conjunctivitis, and 5% for atopic eczema.8–14
The differences in prevalence of asthma in various parts of the world suggest that besides the genetic allergic ground there are also environmental factors, infectious and not infectious, to be studied.15,16 Thus, the increased prevalence of active and passive smoking worldwide is a risk factor for the development of asthma in adolescents.17–21. Other environmental indoor factors for asthma and allergies are: some pollutants, such as ambient particulate matter (PM) that penetrate from the outside, and some are generated and remain indoors, such as particles and gases from smoking, heating, cooking, and cleaning.
Biological contaminants include antigens from house dust mites, moulds, rodents, cockroaches (which are common in Brazil), and animal dander. Dampness and endotoxins have also been implicated in health risks associated with indoor factors.22–24 Meteorological variations and outdoor air pollution, mainly traffic-derived air pollution, not only exacerbate asthma but may also be associated with development of new disease.25 Due to its importance, the role of exposure to animal antigens, although difficult to assess because of different study designs and heterogeneous results,26–30 deserves detailed investigation.
The lack of knowledge about the prevalence of asthma and the importance of associated risk factors in the city of Taubaté were the main reasons for this study, which has the objective of establishing parameters for the control of asthma.
Materials and methodsWe used a cross-sectional study of a random sample probability sample by conglomerates in which the prevalence of asthma was evaluated in 920 students in the city of Taubaté, a middle-sized city 100 miles from São Paulo city, São Paulo State, in Brazil. Adolescents (13-14 years of age) from public and private schools, chosen at random during a period of six months, agreed to participate in the study. The adolescents and/or their parents signed a consent form to participate. Subjects who refused to complete the consent form or the questionnaires were excluded. The age group of 13-14 years was chosen both due to the high asthma-related morbidity8 and so that the adolescents themselves could answer the questionnaires.31 Based on the true prevalence of asthma of nearly 23% in the age group studied,12 and because of the standard error of the estimated prevalence of 1.5%, the sample size was estimated to be 800 students. After considering the possible losses, this number was increased by 15%, with a final sample population consisting of 920 adolescents. The questionnaires applied were: ISAAC (Phase 1) with three modules and the complementary questionnaire with a module on environmental factors. The first three modules based on the ISAAC contained eight questions relating to asthma, six questions related to rhinitis and only one question concerning eczema. The complementary questionnaire contained eight questions about smoking (active and passive) and four questions concerning the presence of animals and/or insects in the home. (Attachment 1) Questionnaires were given to students from selected schools to be completed after the principal investigator had explained both the contents of the questionnaires and the objective of the study. The time needed to complete the questionnaires ranged from 15-20minutes per classroom, and those students that could not fill in the questionnaires completely at school or did not remember all their data, were allowed to finish filling in the questionnaires at home and return them up to one week later, which occurred in 5% of the population.
After tabulating the data using Epi-info version 6.0 and analysing possible differences between genders, the correlations between active asthma and risk factors were tested using a chi-square and/or Fisher's exact test. The critical alpha for determination was 5%. The Ethics Committees of the Universities of São Paulo and Taubaté (SP) approved this protocol and the informed consent form.
ResultsOf the 920 questionnaires distributed, there was a loss of 8.7% (questionnaires not returned and/or incomplete). In the final sample, of the 809 adolescents who met the study criteria, 352 (43.5%) were male and 457 (56.5%) were female. Regarding the type of schools the participants attended, 151 attended private schools (18.8%) and 654 attended public schools (81.2%). The level of maternal education of the participants was 34.5% for primary education, 38.4% for secondary education and 27.1% for higher education. Family income per month ranged from one minimum wage (7.7%) to over 10 times the minimum wage (16%), with a minimum wage of R$260.00 at the time of the survey. The results obtained in the first module (asthma) are shown in Table 1.
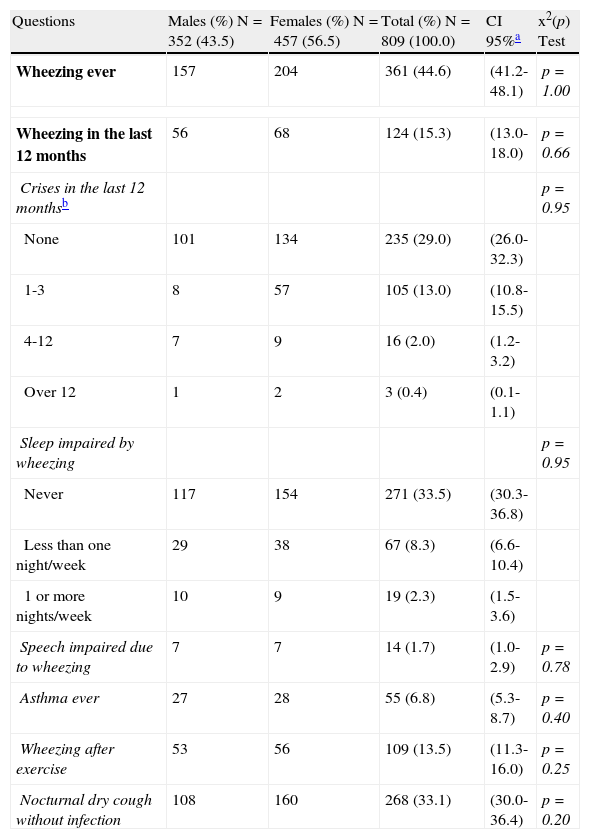
Prevalence rate (%) of asthma and asthma symptoms, by gender.
| Questions | Males (%) N=352 (43.5) | Females (%) N=457 (56.5) | Total (%) N=809 (100.0) | CI 95%a | x2(p) Test |
| Wheezing ever | 157 | 204 | 361 (44.6) | (41.2-48.1) | p=1.00 |
| Wheezing in the last 12 months | 56 | 68 | 124 (15.3) | (13.0-18.0) | p=0.66 |
| Crises in the last 12 monthsb | p=0.95 | ||||
| None | 101 | 134 | 235 (29.0) | (26.0-32.3) | |
| 1-3 | 8 | 57 | 105 (13.0) | (10.8-15.5) | |
| 4-12 | 7 | 9 | 16 (2.0) | (1.2-3.2) | |
| Over 12 | 1 | 2 | 3 (0.4) | (0.1-1.1) | |
| Sleep impaired by wheezing | p=0.95 | ||||
| Never | 117 | 154 | 271 (33.5) | (30.3-36.8) | |
| Less than one night/week | 29 | 38 | 67 (8.3) | (6.6-10.4) | |
| 1 or more nights/week | 10 | 9 | 19 (2.3) | (1.5-3.6) | |
| Speech impaired due to wheezing | 7 | 7 | 14 (1.7) | (1.0-2.9) | p=0.78 |
| Asthma ever | 27 | 28 | 55 (6.8) | (5.3-8.7) | p=0.40 |
| Wheezing after exercise | 53 | 56 | 109 (13.5) | (11.3-16.0) | p=0.25 |
| Nocturnal dry cough without infection | 108 | 160 | 268 (33.1) | (30.0-36.4) | p=0.20 |
Mean prevalence rates and 95% confidence intervals of the results obtained were: 44.6% (41.2-48.1) of adolescents reported “wheezing ever”, 15.3% (13-18) experienced wheezing in the last 12 months (“current asthma”), 15.4%(0.1-15.5) had one or more “wheezing crises” in the last 12 months, 10.6% (1.5-10.4) experienced “impaired sleep due to wheezing”, 1.7% (1-2.9) had “speech impaired by wheeze”, 6.8% (5.3-8.7) had “asthma ever” in life (physician-diagnosed asthma), 13.5% (11.3-16%) showed “wheezing after exercise” (exercise-induced asthma) and 33.1% (30-36.4) had “night cough without infection” (likely nocturnal asthma). There were no significant differences between genders based on the ISAAC questionnaire. The results of the second module (rhinitis) are shown in Table 2.
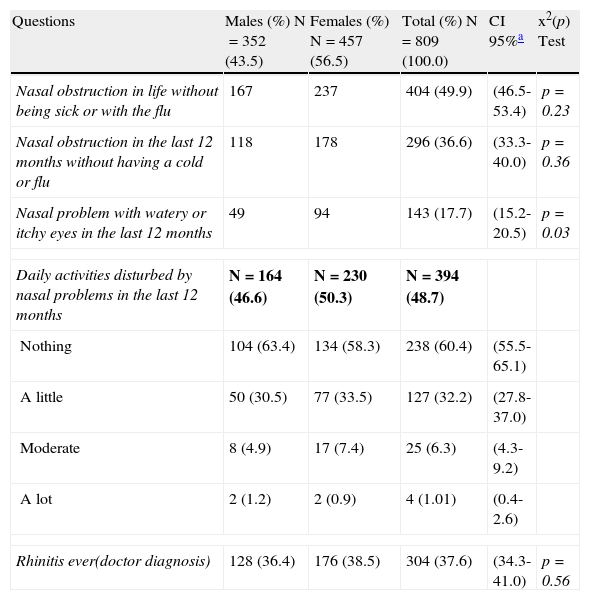
Prevalence rate (%) of allergic rhinitis and rhinitis symptoms, by gender.
| Questions | Males (%) N=352 (43.5) | Females (%) N=457 (56.5) | Total (%) N=809 (100.0) | CI 95%a | x2(p) Test |
| Nasal obstruction in life without being sick or with the flu | 167 | 237 | 404 (49.9) | (46.5-53.4) | p=0.23 |
| Nasal obstruction in the last 12 months without having a cold or flu | 118 | 178 | 296 (36.6) | (33.3-40.0) | p=0.36 |
| Nasal problem with watery or itchy eyes in the last 12 months | 49 | 94 | 143 (17.7) | (15.2-20.5) | p=0.03 |
| Daily activities disturbed by nasal problems in the last 12 months | N=164 (46.6) | N=230 (50.3) | N=394 (48.7) | ||
| Nothing | 104 (63.4) | 134 (58.3) | 238 (60.4) | (55.5-65.1) | |
| A little | 50 (30.5) | 77 (33.5) | 127 (32.2) | (27.8-37.0) | |
| Moderate | 8 (4.9) | 17 (7.4) | 25 (6.3) | (4.3-9.2) | |
| A lot | 2 (1.2) | 2 (0.9) | 4 (1.01) | (0.4-2.6) | |
| Rhinitis ever(doctor diagnosis) | 128 (36.4) | 176 (38.5) | 304 (37.6) | (34.3-41.0) | p=0.56 |
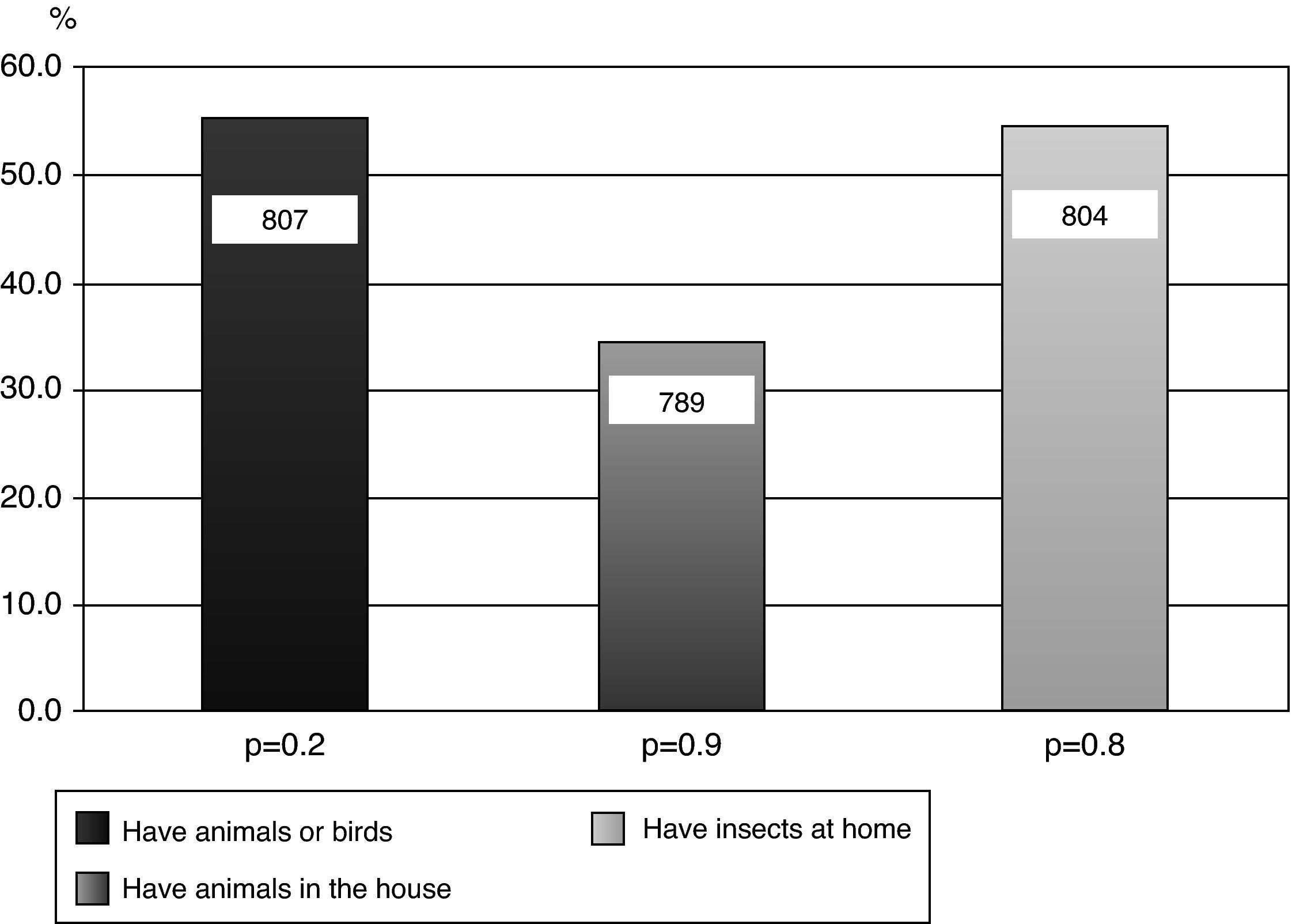
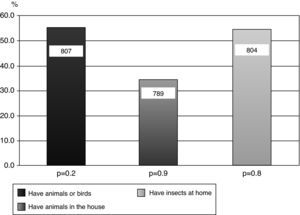
Of the results obtained, 37.6% (34.3-41) reported “rhinitis ever” (diagnosed rhinitis). Among the adolescents who reported having had “asthma ever”, there was a significant association with the response “rhinitis ever” (p=0.02). With regards to the question about “nasal obstruction in the last 12 months accompanied by watery or itchy eyes”, we observed a higher prevalence in females (p=0.03). Of the 809 adolescents studied, 16.2% (15.3-16.8) reported having “eczema ever” (eczema diagnosed by a doctor). Among non-asthmatic children, participants not diagnosed by physician as asthmatics, those who reported “eczema ever” showed a higher chance of having “rhinitis ever” (p=0.04). In the questionnaire regarding smoking, only six adolescents were smokers (0.7%); however, among non-smokers, 3.1% had smoked cigarettes in the past. With regard to smoking in the household, 41% responded yes, with an equal proportion of mothers and fathers who smoked. There was no significant association between current asthma and active smoking in the household (p=0.5). Regarding the presence of animals and insects at home, 55.6% of the 807 adolescents had some form of furry animal or bird, and 34% stated that the animals were kept indoors. There was no significant association between “current asthma” and ownership of an animal or bird (p=0.2), or the presence of animals and/or birds indoors (p=0.9). (Fig. 1) Of the 804 adolescents who answered the question about the presence of cockroaches at home, 55.1% replied affirmatively. However, there was no significant association between current asthma and the presence of insects at home (p=0.8). (Fig. 1)
DiscussionDespite considerable research, the aetiology of asthma and allergic disease remains poorly understood. The International Study of Asthma and Allergies in Childhood (ISAAC), was founded to maximise the value of epidemiological research into asthma and allergic disease by establishing a standardised methodology and facilitating international collaboration. It has achieved its specific aims which are to describe the prevalence and severity of asthma, rhinitis and eczema in children living in different centres and to make comparisons within and between countries. The standardised and coordinated approach of ISAAC enabled the collection of comparable data from children throughout the world, including non-English language populations, and countries in the developing world. Phase One of ISAAC represented by far the most extensive international survey of symptoms of asthma, rhinitis and eczema ever performed.32
The current study showed that the prevalence of “asthma ever” (diagnosed asthma) was 6.8%, which is lower than the prevalence in other regions of the country.8–14 However, it is interesting to note the large difference in the prevalence rates of diagnosed asthma between cities such as Taubaté (6.8%) and Belém (32.8%) which are in very different latitudes of the country.8,12 The prevalence of “current asthma” in adolescents from Brazil ranges from 9.6% (Itabira) to 27.1% (Salvador).9 It is possible that this difference arises from regional differences related to different latitudes, environmental factors, different lifestyles or even different diagnostic criteria.8–12 Another way to assess an asthma diagnosis is to inquire into the presence of wheezing over the past year. It is believed that the period of one year is ideal in order to ensure a clearer memory of events.31 The question “wheezing in the last 12 months” (current asthma) showed a high prevalence (15.3%) in the present study, equivalent to the highest values worldwide. The discrepancy between “asthma ever” and “current asthma” in adolescents in Taubaté suggests a failure to recognise the disease. In addition, it probably demonstrates the need to update health professionals, families and the general population, focusing on high priority actions that must be adopted by the appropriate practitioners for the early diagnosis and control of asthma. A similar phenomenon occurred in other recent national studies.9–14 The significant prevalence of current asthma in the city of Taubaté requires more explanation, besides the habitual genetic, infectious and indoors factors. In this context, the fact that the city of Taubaté is considered to be a great industrial centre raises the hypothesis that environmental pollutants that are not routinely monitored by the Environmental Technology Sanitation Company – CETESB, might contribute to this prevalence because the pollution level reported during the years of this study was low.33
As such, well-established criteria for the diagnosis of asthma are important and should contribute to the better standardisation of preventive measures, medical procedures and performance of health departments, which in turn should minimise the potential risks of morbidity and mortality rates.34,35
Regarding the data on the prevalence rates of rhinitis worldwide, the rates ranged from 1.4-39.7% according to surveys involving 13- to 14-year-old adolescents conducted in 155 cities on several continents.36 A recent study of Brazilian adolescents within this age group which was conducted in 20 regions of Brazil showed a variation in the prevalence of rhinitis from 2.8- 42.1%, with the highest values recorded in Porto Alegre (RS) and Belém (PA).37 In Taubaté, the mean prevalence of diagnosed rhinitis was 37.6% (34.1-41.0), surpassing the national average. This high prevalence of rhinitis may be linked to an allergic background, associated to the “atopic march”. Patients with atopic dermatitis are at increased risk for developing other atopic disorders later in life, including rhinitis and asthma,38 and also to environmental factors such as passive smoking, in which the frequency was elevated in the studied group (41%). We considered that the greater frequency of “nasal obstruction with watery or eye itching” symptoms (rhino-conjunctivitis) in females may reflect different gender lifestyle habits, in which girls spend more time indoors and are exposed to household environmental factors as previously reported.11 Diagnosed eczema rates worldwide range from 1.1-49.3%.39 In Brazil, the highest rates were observed in the north and northeast, with prevalence rates from 2.2 to 14%.40 In Taubaté, the mean prevalence of diagnosed eczema was 16.2% (15.3-16.8). As for the diagnosis of rhinitis, we consider an allergic background38 and also environmental factors to be the likely causes for this high prevalence. A study carried out in Spain showed that smoking by parents was considered a risk factor for developing eczema in 6- to 7-year-old children but not in the 13- to 14-year-old population.41 We suggest that the high prevalence of current asthma, rhinitis and eczema in this study, resting upon genetic foundations of our population of adolescents, could have been influenced by some environmental factors and local pollutants, which justifies further research. Some studies in Brazil have shown that the prevalence of active smoking in adolescents is increasing in Brazil.19,20 The frequency of active smoking in our study was lower (0.7%) than that reported in other national studies, even when using a similar questionnaire for evaluation.19,20 We believe that 5% of our population could have provided incorrect data because they had taken the questionnaire at home. Parental smoking is commonly associated with asthma symptoms, respiratory infections and frequent hospitalisations in children.21 A study conducted in Pouso Alegre (Minas Gerais) showed a 1.5-fold higher risk of “wheezing in the last 12 months” among adolescents who had contact with smokers at home.13 In this study, there was no association between the presence of passive smoking and current asthma, findings that agree with those of Maia et al. (2004).12
Sensitisation to animal antigens is a risk factor for asthma.23 The relationship between exposure to domestic animals during childhood, development of sensitisation and asthma symptoms is still complex.26–30 Data regarding sensitisation in animal owners are heterogeneous; some studies have suggested that having a pet increases the risk of sensitisation27 while others suggest that it decreases the risk.28,29 In the present study of 807 adolescents, 55.6% owned animals and/or birds and 34% reported that these animals were kept indoors. However, there was no statistically significant correlation between “wheezing in the last 12 months” and the presence of animals indoors (p=0.9). It has been argued that the different asthma phenotypes are defined by genetic and environmental factors and that individual responses to antigens and sensitisation depend on antigenic load and exposure time.28
Sensitisation to cockroaches is common in children with perennial asthma and is related to more severe forms of the disease.24 Cutaneous tests in atopic children and those with asthma vary in frequency from 24.1-32.8% in diverse Brazilian cities.23,24 In the current study, 55.1% of adolescents reported the presence of cockroaches at home, but no statistically significant relationship was found between “wheezing in the last 12 months” and the presence of these insects at home (p=0.8). However, other studies have shown a positive association between the presence of cockroaches in the home and asthma-related symptoms.23
Clearly, the report of whether or not there are cockroaches in a house is not enough to correlate asthma and sensitisation to the antigens of these insects in our study. The inclusion of cutaneous tests to better evaluate the correlation between asthma and exposure to cockroach antigens should be considered in future research in the city of Taubaté.
In this study, we opted to describe the presence of risk factors in patients with current asthma over the past 12 months because it provides reliable data and helps prevent errors of memory in the adolescents studied.31
The lack of a correlation between current asthma and the frequency of risk factors studied in this group may have occurred due to the duration and intensity of exposure, differences in the care for animals and hygiene and also variables that were not addressed in the current study.
In conclusion, the prevalence of current asthma in 13- to 14-year-old adolescents attending schools in the city of Taubaté was relatively high from an epidemiological point of view, affecting approximately 1 in 15 adolescents. However, when compared to other studies in Brazil (ranging from 1.8 to 30.2% when similar methodology is applied), the prevalence of diagnosed asthma of 6.8% can be considered relatively low, accounting for about one-third of the values observed in most studies. Contrary to that observed for asthma, the prevalence of diagnosed rhinitis and eczema was high and well above the prevalence described in most other similar studies conducted in other regions of the country. Finally, with regard to risk factors commonly cited in the literature, a significant association was not observed in our patients with current asthma in Taubaté.
Conflict of interestThe authors declare no conflict of interest.
We would like to thank Dr. Ciro João Bertoli for facilitating the access to public and private entities, thus allowing for the study to be performed, for justifying the absence from teaching activities when necessary and for offering relevant suggestions for the final outcome of the research.