There are no country-based data focused on aspirin (ASA)-exacerbated respiratory disease (AERD) in Turkey.
ObjectiveTo assess the prevalence of AERD in adult patients with asthma.
MethodsA structured questionnaire was administered via face-to-face interview by a specialist in pulmonology/allergy at seven centres across Turkey.
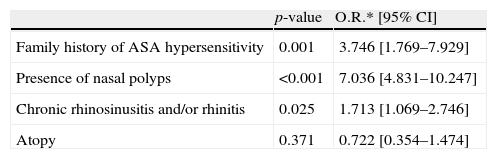
ResultsA total of 1344 asthma patients (F/M: 1081/263: 80.5%/19.5%, mean age: 45.7±14.2 years) were enrolled. Atopy rate was 47%. Prevalence of allergic rhinitis, chronic rhinosinusitis/rhinitis, and nasal polyposis (NP) were 49%, 69% and 20%, respectively. Of 270 patients with NP, 171 (63.3%) reported previous nasal polypectomy and 40 (25%) had a history of more than three nasal polypectomies. Aspirin hypersensitivity was diagnosed in 180 (13.6%) asthmatic patients, with a reliable history in 145 (80.5%), and oral ASA provocation test in 35 (19.5%) patients. Clinical presentations of ASA hypersensitivity were respiratory in 76% (n=137), respiratory/cutaneous in 15% (n=27), and systemic in 9% (n=16) of the patients. Multivariate analysis indicated that a family history of ASA hypersensitivity (p: 0.001, OR: 3.746, 95% CI: 1.769–7.929), history of chronic rhinosinusitis/rhinitis (p: 0.025, OR: 1.713, 95% CI: 1.069–2.746) and presence of NP (p<0.001, OR: 7.036, 95% CI: 4.831–10.247) were independent predictors for AERD.
ConclusionThis cross-sectional survey showed that AERD is highly prevalent among adult asthmatics and its prevalence seems to be affected by family history of ASA hypersensitivity, history of rhinosinusitis and presence of NP.
A variety of hypersensitivity reactions, varying from cutaneous reactions to severe systemic reaction, related with aspirin (ASA) consumption have been frequently reported.1 The prevalence of ASA hypersensitivity changes with the method used for the diagnosis, however, a group of chronic diseases such as nasal polyp (NP), asthma and chronic urticaria are associated with a higher rate of ASA hypersensitivity than in the general population.2
ASA-exacerbated respiratory disease (AERD) is a distinct, clear-cut phenotype of asthma. The disease is characterised by a natural sequence of symptoms: first rhinitis related by most patients to a flu-like infection, then perennial eosinophilic rhinosinusitis/NP, followed by ASA-induced respiratory reaction. Precipitation of asthmatic attacks by ASA and other non-steroidal anti-inflammatory drugs (NSAIDs) that inhibit cyclooxygenase (COX)-1 constitute a hallmark of this clinical syndrome. The disease runs a protracted, usually severe course with about half of the patients requiring at least bursts of corticosteroids to control their rhinosinusitis and asthma.3 The prevalence of AERD in adult asthmatic patients ranges from 1% to 20%.1–4 A few studies have reported data regarding the frequency of ASA hypersensitivity, asthma and related issues in our country.5–7 However, these trials were single-centre based and none of them specifically targeted the prevalence of AERD among the adult population with asthma. Consequently, there has been no nationwide epidemiological study on the prevalence of AERD in Turkey. Therefore, in this multi-centre study, we first aimed to assess the prevalence of AERD in adult asthmatics, and secondarily aimed to document the clinical features of the disease and risk factors for our population.
Material and methodsPatient selectionThis prospective, national and multi-centre study was conducted at seven different tertiary healthcare centres across Turkey. The tertiary healthcare centres for chest and allergic diseases were selected to be representative of the country according to the geographical distribution of specialists in Turkey as well as the distribution of investigators in relation to the type of institutions in which they work (university hospital, state chest diseases hospital). All adults with asthma were prospectively included in the study throughout 2007. The patients were assured of the voluntary nature of the study, and all gave verbal informed consent. The patients were regularly visiting their physicians and were on health care cover. Asthma diagnosis was based on criteria defined by the American Thoracic Society.8 The diagnosis of NP and rhinosinusitis was based on visualisation of bilateral polyps in nasal cavities by either endoscopic examination and/or by a CT scan of the paranasal sinuses, in addition to compatible history. The asthma control level was classified as controlled, partly controlled, and uncontrolled by means of physician assessment considering daytime/night-time symptoms, limitations of activities, need for rescue treatment, and the number of exacerbations, based on the Global Initiative for Asthma (GINA) guidelines.9
QuestionnaireA specifically designed questionnaire was developed by the authors and used via face-to-face interview by a specialist in pulmonology and allergy to evaluate the prevalence of AERD. It consisted of questions about the patients’ demographics including age, gender, the duration of asthma, NP, rhinosinusitis/rhinitis, medications, and emergency room visit/hospitalisation for the preceding year. Additional items concerning the number of nasal polypectomies, family history of ASA respiratory hypersensitivity, and reactions to antibiotics or other drugs were included. Diagnosis of ASA hypersensitivity was based on either the patient's history and/or oral ASA provocation. Aspirin hypersensitivity was established by six questions including if there had ever been any reaction after ASA and/or other NSAIDs, the number of reactions, and details of the symptoms, which occurred following these medications. Nasal congestion/rhinorrhea, and/or shortening of breath and rapidly progressing bronchial obstruction within 30–120min following ingestion of ASA and/or other COX-inhibiting NSAIDs was considered a positive history for ASA hypersensitivity. Other extrabronchial symptoms including ocular, cutaneous or gastric symptoms accompanying respiratory symptoms were also accepted as being positive for ASA hypersensitivity. In those centres, which had experienced personnel and necessary equipment, oral ASA provocation was performed to confirm the diagnosis of ASA hypersensitivity. An oral provocation test with ASA was performed with the method previously described.10
Evaluation of atopyAtopy was defined as a positive skin prick (a mean diameter of 3mm greater than the negative control) and/or specific IgE (>0.35kU/l with ImmunoCAP system, Phadia, Uppsala, Sweden) to at least one of the aeroallergens. Glycerinated extracts of Dermatophagoides pteronyssinus, Dermatophagoides farinae, cockroach, grass, tree, weed pollens, cat, dog, and Alternaria and Cladosporium antigens (Stallergenes, Antony/France or ALK-Abello, Madrid/Spain) were used along with positive and negative controls.
StatisticsNumerical values are given as mean±SEM and categorical/ordinal values as n (%). Numerical values and ordinal/categorical values of patients with AERD and patients with ASA-tolerant asthma were compared by unpaired samples T-test and Chi-square tests, respectively. A p-value less than 0.05 were considered as statistically positive. The significant factors obtained in the univariate analysis in the comparison of both groups were re-formulated for a multivariate logistic regression model. Data were given as odds ratio (OR) and 95% confidence interval.
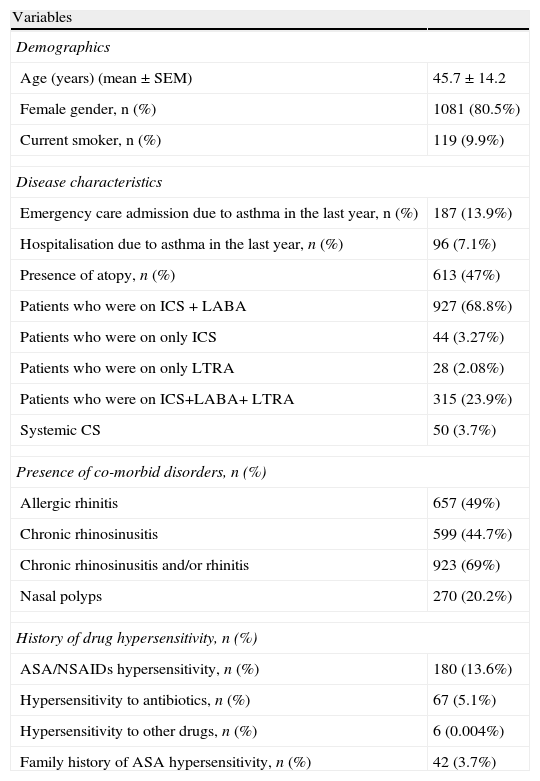
ResultsA total of 1344 patients with a mean age of 45.7±14.2 years were enrolled in this study (Table 1). Females were predominant (80.5%) and nearly half of the patients were atopic (47%). Mite was the most common sensitising allergen (n: 193, 31.8%), followed by pollens (n: 147, 24.3%), pollen plus mite (n: 184, 30.4%), moulds (n: 20, 3.3%). Of all patients, 829 patients (67.3%) had controlled (one or less than twice daily symptoms without any nocturnal symptom, need for rescue medication, activity limitation, and exacerbation), 268 (21.8%) had partly controlled, and 134 (10.9%) had uncontrolled asthma. The majority of patients (71%) were identified to be on combined drug treatment, either inhaled corticosteroids (ICS) and long acting beta-2 agonist: (LABA) in 927 patients (68.9%), or ICS+LABA+leukotriene receptor antagonist (LTRA) in 315 patients (23.9%). Fifty patients (3.7%) were on a systemic steroid, and 15 patients (1.16%) were using theophylline. A few patients were on only one controller such as ICS in 44 patients (3.27%), and LTRA in 28 patients (2.08%) (Table 2).
Demographics and disease characteristics of the study population (n=1344).
| Variables | |
| Demographics | |
| Age (years) (mean±SEM) | 45.7±14.2 |
| Female gender, n (%) | 1081 (80.5%) |
| Current smoker, n (%) | 119 (9.9%) |
| Disease characteristics | |
| Emergency care admission due to asthma in the last year, n (%) | 187 (13.9%) |
| Hospitalisation due to asthma in the last year, n (%) | 96 (7.1%) |
| Presence of atopy, n (%) | 613 (47%) |
| Patients who were on ICS + LABA | 927 (68.8%) |
| Patients who were on only ICS | 44 (3.27%) |
| Patients who were on only LTRA | 28 (2.08%) |
| Patients who were on ICS+LABA+ LTRA | 315 (23.9%) |
| Systemic CS | 50 (3.7%) |
| Presence of co-morbid disorders, n (%) | |
| Allergic rhinitis | 657 (49%) |
| Chronic rhinosinusitis | 599 (44.7%) |
| Chronic rhinosinusitis and/or rhinitis | 923 (69%) |
| Nasal polyps | 270 (20.2%) |
| History of drug hypersensitivity, n (%) | |
| ASA/NSAIDs hypersensitivity, n (%) | 180 (13.6%) |
| Hypersensitivity to antibiotics, n (%) | 67 (5.1%) |
| Hypersensitivity to other drugs, n (%) | 6 (0.004%) |
| Family history of ASA hypersensitivity, n (%) | 42 (3.7%) |
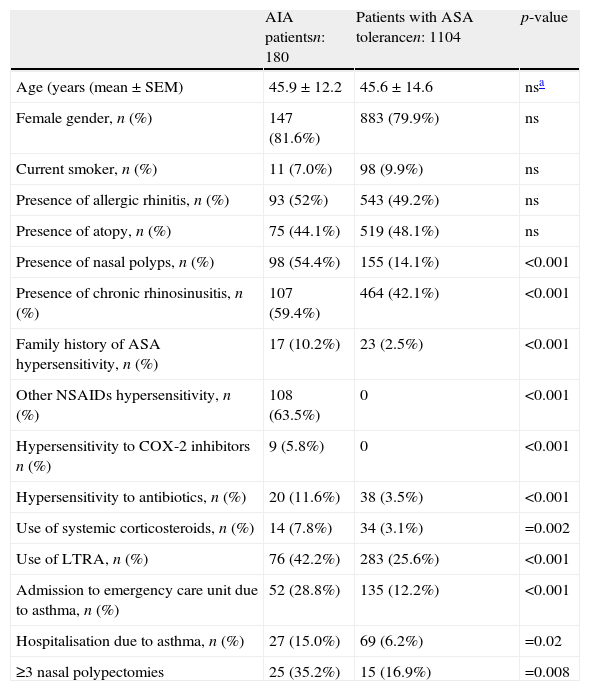
Comparison of asthma patients with or without ASA hypersensitivity.
| AIA patientsn: 180 | Patients with ASA tolerancen: 1104 | p-value | |
| Age (years (mean±SEM) | 45.9±12.2 | 45.6±14.6 | nsa |
| Female gender, n (%) | 147 (81.6%) | 883 (79.9%) | ns |
| Current smoker, n (%) | 11 (7.0%) | 98 (9.9%) | ns |
| Presence of allergic rhinitis, n (%) | 93 (52%) | 543 (49.2%) | ns |
| Presence of atopy, n (%) | 75 (44.1%) | 519 (48.1%) | ns |
| Presence of nasal polyps, n (%) | 98 (54.4%) | 155 (14.1%) | <0.001 |
| Presence of chronic rhinosinusitis, n (%) | 107 (59.4%) | 464 (42.1%) | <0.001 |
| Family history of ASA hypersensitivity, n (%) | 17 (10.2%) | 23 (2.5%) | <0.001 |
| Other NSAIDs hypersensitivity, n (%) | 108 (63.5%) | 0 | <0.001 |
| Hypersensitivity to COX-2 inhibitors n (%) | 9 (5.8%) | 0 | <0.001 |
| Hypersensitivity to antibiotics, n (%) | 20 (11.6%) | 38 (3.5%) | <0.001 |
| Use of systemic corticosteroids, n (%) | 14 (7.8%) | 34 (3.1%) | =0.002 |
| Use of LTRA, n (%) | 76 (42.2%) | 283 (25.6%) | <0.001 |
| Admission to emergency care unit due to asthma, n (%) | 52 (28.8%) | 135 (12.2%) | <0.001 |
| Hospitalisation due to asthma, n (%) | 27 (15.0%) | 69 (6.2%) | =0.02 |
| ≥3 nasal polypectomies | 25 (35.2%) | 15 (16.9%) | =0.008 |
ASA: aspirin; NSAIDs: nonsteroidal anti-inflammatory drugs; LTRA: leukotriene receptor antagonist; COX: cyclooxygenase.
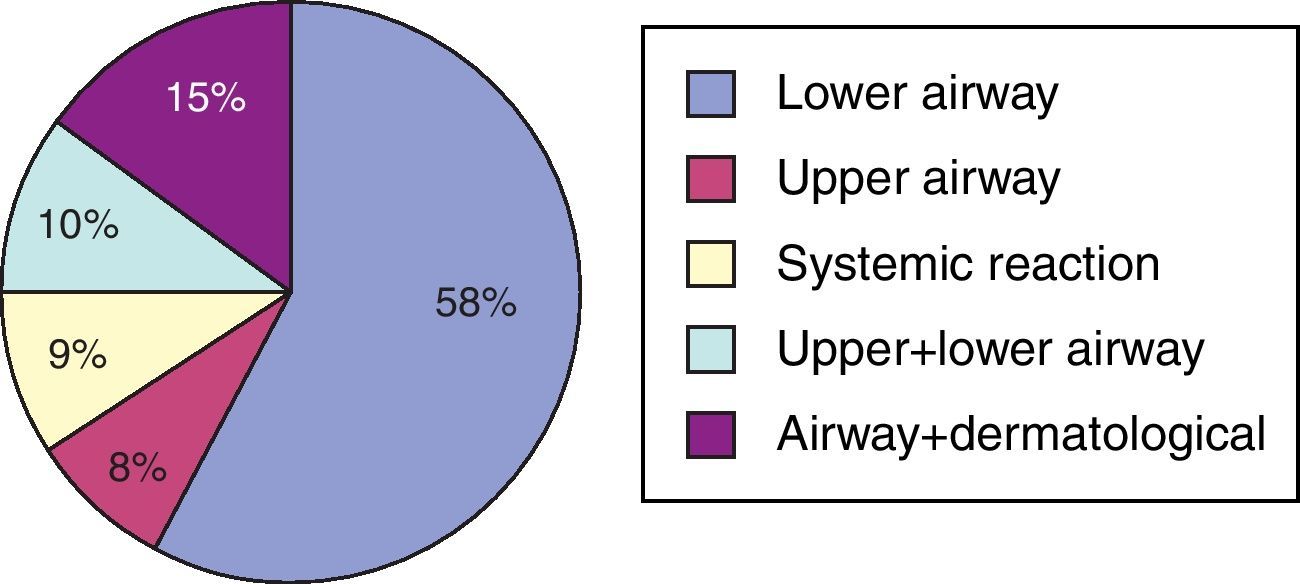
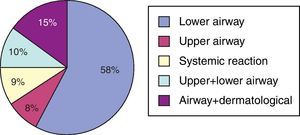
A total of 180 (13.6%) asthma patients were diagnosed to be AERD. The diagnosis of ASA hypersensitivity was based on reliable history in 145 cases (80.5%), and ASA provocation was performed in 35 (19.5%) patients. Eight patients among the ASA-tolerant group were challenged with ASA, and two resulted in positive. In contrast, there were 4 patients who were negative to ASA provocation test among patients with a positive history to ASA and challenged with ASA. Aspirin was the major NSAID causing hypersensitivity reactions in 149 (82.7%) patients, and 108 (63.5%) patients reported hypersensitivity reaction to other NSAIDs in addition to ASA. In 31 (17.3%) of 180 patients with AERD, reactions were precipitated only by other NSAID, 9 (5%) reacted to COX-2 inhibitors, and 20 (11.6%) reacted to antibiotics. Clinical presentations of ASA hypersensitivity were lower respiratory in 58% (n=105), upper respiratory in 8% (n=14), upper and lower respiratory in 10% (n=18), respiratory and cutaneous in 15% (n=27), and systemic type in 9% (n=16) of the patients (Fig. 1).
In the comparison of patients with AERD and ASA tolerant asthma, there were no significant differences in term of age, gender, smoking rate, presence of allergic rhinitis, and atopy between the groups. There was no difference in asthma control levels between AERD and ASA tolerant asthma (p>0.05). The ratio of the patients with controlled, partly controlled and uncontrolled asthma were 63.3% (n=112), 23.2% (n=41) and 13.6% (n=24), respectively, among the patients with AERD. Similarly, the ratio of patients with controlled, partly controlled, and uncontrolled asthma were 68% (717), 21.5% (n=227) and 10.4% (n=110), respectively, among the ASA tolerant group. However, AERD was significantly associated with the presence of NP, chronic rhinosinusitis/rhinitis, family history of ASA hypersensitivity, hypersensitivity to COX-2 inhibitors and antibiotics, the use of systemic steroid and LTRA, emergency department visits and hospitalisation due to asthma during the past year (p<0.05, p<0.001). The relationship between ASA hypersensitivity and numbers of polypectomies was statistically significant (p=0.016). The rate of three or more nasal polypectomies history was 35.2% in patients with AERD. This rate was 16.9% in patients not having ASA hypersensitivity (p<0.008). A total of 270 patients were diagnosed as having NP. Of them, 171 (63.3%) reported previous nasal polypectomy, and 40 (25%) had a history of more than three nasal polypectomies.
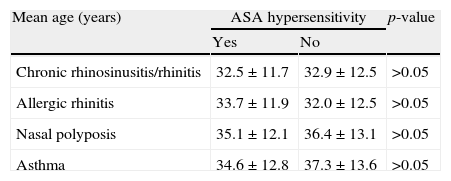
When we compared the patients who are ASA tolerant or have AERD, all were in their early thirties and there was no statistically significant difference between mean ages at the onset of chronic rhinosinusitis/rhinitis, allergic rhinitis, NP and asthma (p>0.05) (Table 3).
Mean age at onset of accompanying diseases according to ASA hypersensitivity.
| Mean age (years) | ASA hypersensitivity | p-value | |
| Yes | No | ||
| Chronic rhinosinusitis/rhinitis | 32.5±11.7 | 32.9±12.5 | >0.05 |
| Allergic rhinitis | 33.7±11.9 | 32.0±12.5 | >0.05 |
| Nasal polyposis | 35.1±12.1 | 36.4±13.1 | >0.05 |
| Asthma | 34.6±12.8 | 37.3±13.6 | >0.05 |
Multivariate analysis indicated that family history of ASA hypersensitivity (p: 0.001, OR: 3.746, 95% CI: 1.769–7.929), history of rhinosinusitis/rhinitis 1.713 (p: 0.025, OR: 1.713 95% CI: 1.069–2.746) and presence of nasal polyposis (p<0.001, OR: 7.036, 95% CI: 4.831–10.247) were independent predictors for ASA hypersensitivity in patients with asthma (Table 4).
Results from multiple logistic regression analysis in favour of ASA hypersensitivity.
| p-value | O.R.* [95% CI] | |
| Family history of ASA hypersensitivity | 0.001 | 3.746 [1.769–7.929] |
| Presence of nasal polyps | <0.001 | 7.036 [4.831–10.247] |
| Chronic rhinosinusitis and/or rhinitis | 0.025 | 1.713 [1.069–2.746] |
| Atopy | 0.371 | 0.722 [0.354–1.474] |
The present study demonstrated that the frequency of AERD was 13.6% in our country and its prevalence appeared to be affected by a family history of ASA hypersensitivity, the presence of NP, and chronic rhinosinusitis/rhinitis.
The exact prevalence of AERD is unknown. A limited number of epidemiological studies reported that the prevalence of AERD ranges from 4% to 44%.3,11 This difference seems to be related with the heterogeneity of the population studied, as well as the methods and criteria used for determining ASA hypersensitivity. A recent systematic review reported that the pooled incidence of AERD is 21% (95% CI, 14% to 29%).4
Most studies about the prevalence of ASA hypersensitivity have been on selected populations, such as patients with severe asthma or patients with required mechanical ventilation.4,12 Very few and reliable studies have targeted the frequency of ASA hypersensitivity in unselected asthma patients.13–16 In a large survey of a population-based random sample (n: 4300) in Finland, the prevalence of ASA hypersensitivity was 1.2% using a postal questionnaire but it was higher in patients with doctor-diagnosed asthma (8.8%).13 In an Austrian postal questionnaire survey, the prevalence of physician diagnosed-AERD was 10–11%.14 More recently, in the largest random selected population study from Poland (n: 12,971), hypersensitivity to ASA/NSAIDs was observed in 12.9% of asthmatics via interview of subjects by medical students and nurses.15,16 We also worked with an unselected outpatient asthma population.
The use of history alone in the assessment of ASA hypersensitivity among asthma patients has been associated with underestimation or overestimation of the real prevalence of AERD.4,17 A recent systematic review reported that history alone resulted in a much lower prevalence (2.7%) compared to the pooled incidence of AERD as 21%.4 Lack of awareness of the patients, who are hypersensitive to ASA, or patients with asthma having been advised to avoid ASA may all contribute to the underdiagnosis of ASA hypersensitivity.17 Furthermore, a recent review4 showed that analyses based on the use of a questionnaire in doctor-diagnosed asthma resulted in a higher number of positive results for AERD (11–24%) than those based on the retrospective analysis of medical records (2–3%).
When ASA provocation is performed, the prevalence of AERD can be as high as 21%.4,12,17 However, because of the potential for severe reactions, provocation tests are not a usual method used for studies of AERD. ASA provocation could be done in five centres in our trial. Since it was not systematically used in all patients in the study, we did not separately evaluate the prevalence of AERD in these centres.
The typical patient with AERD is an adult who develops rhinosinusitis in the third-fourth decade of life. The typical clinical picture is completed with the addition of asthma and ASA hypersensitivity.17 In our trial, the mean ages of the patients at the onset of rhinosinusitis, NP, asthma, and ASA hypersensitivity were around the third decade, and the order of development of AERD was similar to that previously indicated.17
It is well known that AERD patients suffer from a more severe phenotype of persistent asthma than average with higher medication requirements.17–19 Supporting this, our patients with AERD were associated with a higher rate of systemic steroid and LTRA use in addition to ICS+LABA when compared to ASA tolerant patients. Similarly, as reported previously,20,21 a high rate of emergency room visit/hospitalisation was associated with ASA hypersensitivity in our group.
Several risk factors have been supposed for AERD including atopy, persistent rhinosinusitis and NP.22,23 The role of atopy as a risk factor is controversial3,23,24. In this trial, the atopy rate of the whole group was significantly higher than that of the general adult population in Turkey (47% vs. 25%, p>0.05),25 but it seems to be related with the presence of asthma rather than ASA hypersensitivity since the atopy ratio was not different in AERD than in ASA tolerant asthma.
The prevalence of ASA hypersensitivity in patients with NP has been found to be 20–40%.3,26,27 Consistently, in our study, the presence of NP was a significant and independent risk factor for AERD. Moreover, NP has a high tendency for recurrence.22 Likewise, there was a statistically significant (p=0.016) relationship between ASA hypersensitivity and the number of polypectomies in our patients.
Cross-sensitivity to NSAIDs is a well-known feature of ASA hypersensitivity3,4 and in our study 63.5% of patients with AERD also reported reactions to other COX-1 inhibitors. COX-2 inhibitors are generally considered safe in AERD, but some cutaneous and systemic reactions have been reported with them.28,29 In our trial a few (5%) AERD patients had a history of reaction to COX-2 inhibitors; unfortunately we do not have data about the type of reaction to these drugs.
Drug reactions can aggregate in families.30 Familial aggregation of ASA hypersensitivity was reported by 10.2% of our patients with AERD. As far as we know, there are no data regarding familial aggregation of ASA hypersensitivity with the exception of a multicentre study in which the family history of hypersensitivity to ASA has been reported by 6% of the families.17 As a limitation of our study, we did not confirm ASA hypersensitivity in family members. Individuals with a history of ASA hypersensitivity may be more likely to remember ASA hypersensitivity in family members or inadvertent interviewer bias could have influenced the results. A recent study evaluating familial aggregation of ASA-induced urticaria demonstrated LTC4S)-444C alleles aggregations in family members.31 Contrary to studies genetic background of AERD,32–34 there is no genetic study regarding familial aggregation of AERD. It would be interesting to see if there is any inheritance pattern in such patients.
Another limitation of our study is the diagnosis of ASA hypersensitivity, which was mainly based on a detailed history. However, all patients were both recruited from centres, which specialise in allergy/pulmonology, and were also evaluated by specialists. A significant correlation between a history of ASA hypersensitivity and the result of oral challenge has been previously demonstrated.35,36 Therefore, despite this limitation, this questionnaire-based study provides nationwide data regarding the prevalence of AERD in our country for the first time.
In conclusion, this nationwide survey showed that AERD is highly prevalent among our adult asthmatics and its prevalence seems to be affected by family history of ASA hypersensitivity, presence of NP, chronic rhinosinusitis and/or rhinitis.
Conflict of interestThe authors have no conflict of interest. There is no financial support.