Asthma is a chronic, inflammatory disease of the airway, and adrenomedullin (ADM) may have some effects against bronchoconstriction. However, the role(s) of ADM in asthmatic children have not been evaluated yet. The aims of this study were to determine if there are any changes in plasma ADM levels during acute asthma attack, and to search for any association between allergen sensitivity and ADM level in asthmatic children.
MethodsTwenty-seven children with acute asthma attack, ranging in age from 5 to 15 years were investigated and compared with 20 controls. Plasma ADM levels (ng/mL) were measured by ELISA method.
ResultsNo significant difference was found in ADM levels between the controls and patients in either the acute attack or remission period. Plasma ADM levels were significantly higher in the acute attack (p=0.043) compared to the remission period in patients who were considered as having a “severe attack” according to GINA (Global Initiative for Asthma) classification. There were statistically significant correlations between the patients’ AlaTOP and Food Panel 7 levels and plasma ADM levels in the acute attack period (p=0.010, p=0.001, respectively). The ADM levels in patients with a history of atopic dermatitis were significantly higher in the acute attack period compared to those without a history of atopic dermatitis (p=0.007).
ConclusionWe speculate that ADM may have a role in children with atopic dermatitis, and may also have a role in the immuno-inflammatory process of asthma.
Adrenomedullin (ADM) is a peptide consisting of 52 amino acids, and structurally it resembles peptides associated with the calcitonin gene.1 Although it is basically synthesized in phaeochromocytoma cells, it presents in the adrenal medulla, heart, lungs, and kidneys, and in addition to plasma and urine, it is also synthesized and released from the endothelium, vascular smooth muscle cells, myocardium, fibroblasts, and cancer cells.1–4 In experimental studies in rats, it has been shown that ADM causes a long-term bronchodilator effect against the bronchoconstriction induced by histamine and acetylcholine injection.5 The presence of ADM in the bronchial epithelium was shown for the first time in 1995.6 That study demonstrated that tumour necrosis factor (TNF)-alpha yielded an increase in ADM levels, while dexamethasone did not have any effect.6
Asthma is a chronic inflammatory airway disease, in which several cell types and cell components play many roles.7 In an adult study, it has been shown that plasma ADM levels increase in an acute asthma attack.8 However, the effects of ADM in the regulation of the bronchial system in children have not been evaluated thus far. The aims of the present study were to determine whether there are any changes in plasma ADM levels during acute asthma attack in children with bronchial asthma and to search for any association between allergen sensitivity and ADM level.
MethodsSubjectsTwenty-seven children (13 male, 14 female) followed up in our paediatric allergy clinic with acute asthma attack symptoms, ranging in age from 5 to 15 years, were investigated and compared with 20 healthy (10 male, 10 female), age- and sex-matched controls. The mean age of the patients was 8.89±2.90 years.
The study was approved by the Local Ethics Committee of Gaziantep University. An informed consent form was obtained from all patients and healthy controls.
Blood samples were collected before corticosteroid administration in acute attack, and one month later in remission periods in all patients. Children with a raised temperature or clinical infection were excluded. Patients with hypertension and/or renal dysfunction (serum creatinine>1.0mg/dL, creatinine clearance<80mL/min per 1.73m2) were also excluded because both of them can affect plasma and urinary levels of AM.9 Control subjects were free of kidney and chronic disease when enrolled from the general paediatric outpatient clinic. All had a complete physical examination, normal blood urea nitrogen, creatinine and electrolyte levels, and were normotensive at the time of study. Blood samples were taken once in controls. Pulmonary function test and skin prick tests (SPT) had been administered to all patients in the study group. The SPTs were carried out by the same investigator. Commercially available multiheaded devices Quintest (HollisterStier) were used to prick the epidermis through the allergen extract drops. For the SPT, Skin Prick Test panel ALK-Abello allergens were used (3 trees mix, grass mix, weed mix, grain pollen, cladosporium, olea, latex, Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blattella germanica, cat fur, dog hair, Alternaria, feather mix, Aspergillus, egg white). The histamine solution and a saline solution were used as controls. The results were considered positive if the wheal had a mean diameter ≥3mm greater than that of negative control. Specific IgE levels [AlaTOP (D. pteronyssinus cat epithelium), dog dander, Bermuda grass, Timothy grass, Penicillium notatum, Alternaria tenuis, birch, Japanese cedar, common ragweed, English plantain, Parietaria officinalis, and Food Panel 7 (egg white, milk, wheat, rice, peanut, soybean)] were studied using the IMMULITE 2000 device.
Determination of adrenomedullin levelBlood samples were collected into the Lavender Vacutainer tubes which contain EDTA and gently rocked several times immediately after collection of blood for anti-coagulation. Collected blood was transferred from the lavender vacutainer tubes to the centrifuge tubes containing aprotinin (0.6TIU/ml of blood) and gently rocked for several times to inhibit the activity of proteinases. Blood samples were centrifuged at 1600×g for 15min at 4°C and the plasma was collected. The samples were kept at −80°C until the study day. Plasma ADM level (ng/mL) was measured with Dynex-DSX/Virion Serion/Organtec (USA, Catalog number: EK-010-01) by ELISA method. ELISA kit was obtained from Phoenix Pharmaceuticals, Inc (USA).
Statistical analysisResults are provided as means±standard deviation (SD). Differences between groups were compared by the Wilcoxon–Rank and Mann–Whitney U tests for the dependent and independent groups, respectively. A level of p<0.05 was considered to be statistically significant. The statistical analysis software package SPSS 10.0 was used for the analyses.
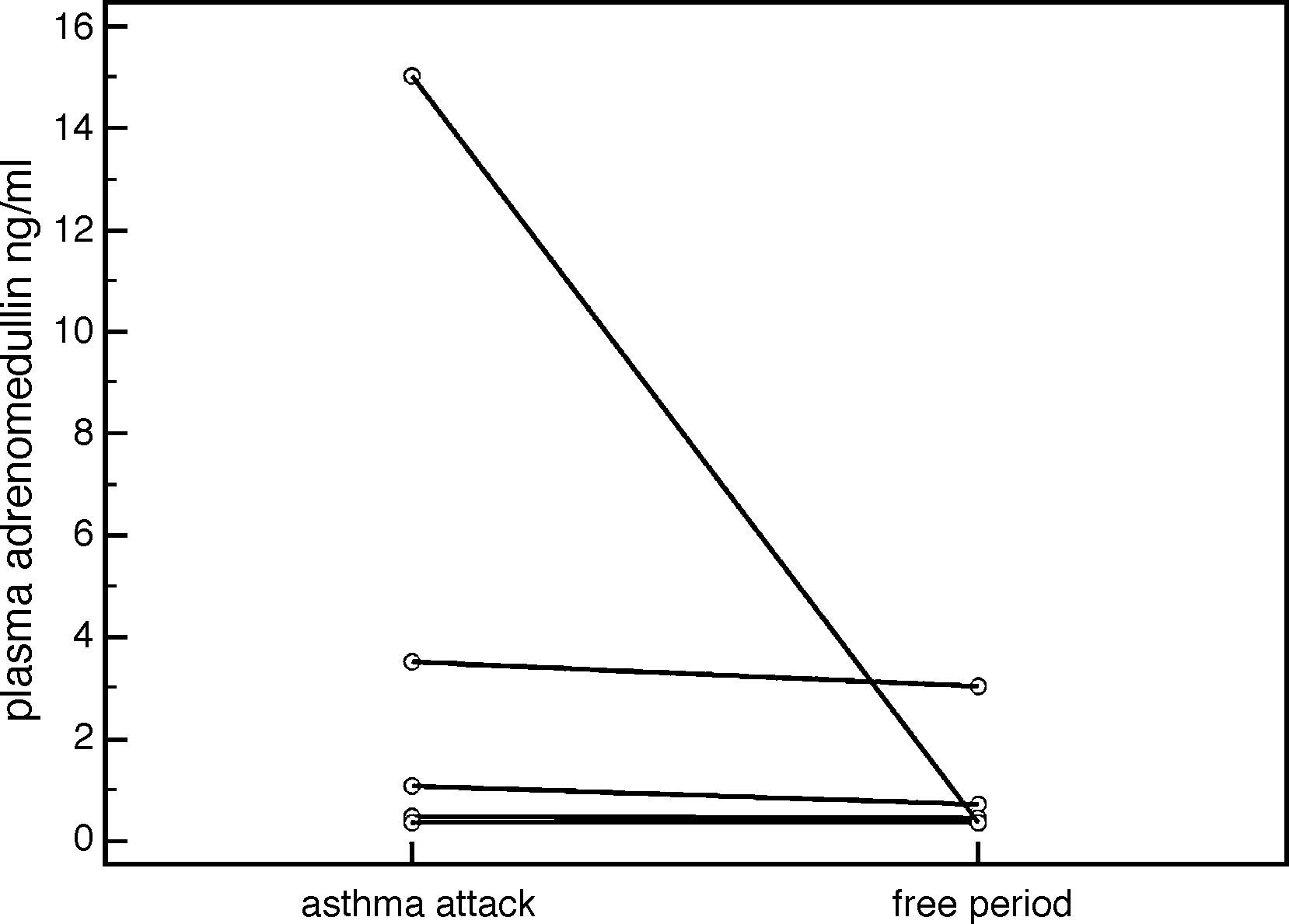
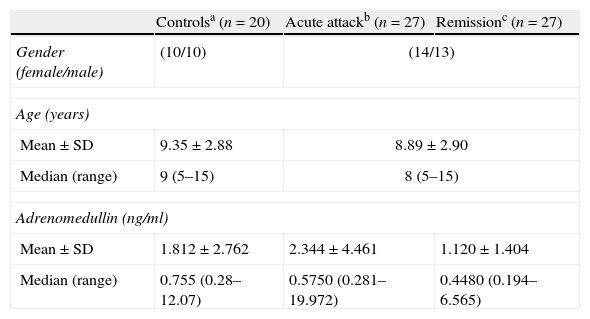
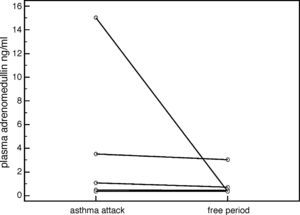
ResultsThere were no statistically significant differences in plasma ADM levels (ng/ml) between the acute attack and remission periods in the patient group (2.344±4.461 vs. 1.120±1.404, respectively, p=0.159) (Table 1). However, plasma ADM levels were significantly higher in the acute attack (3.489±5.775), compared to remission (0.897±1.054) period in patients who were considered as having a “severe attack” according to GINA (Global Initiative for Asthma) classification (p=0.043) (Fig. 1). No significant difference was found in ADM levels between the controls and patients in either the acute attack (1.812±2.762 vs. 2.344±4.461, respectively, p=0.788) or remission period (1.812±2.762 vs. 1.120±1.404, respectively, p=0.22) (Table 1).
Demographic features and adrenomedullin levels of patients with asthma, and controls.
| Controlsa (n=20) | Acute attackb (n=27) | Remissionc (n=27) | |
| Gender (female/male) | (10/10) | (14/13) | |
| Age (years) | |||
| Mean±SD | 9.35±2.88 | 8.89±2.90 | |
| Median (range) | 9 (5–15) | 8 (5–15) | |
| Adrenomedullin (ng/ml) | |||
| Mean±SD | 1.812±2.762 | 2.344±4.461 | 1.120±1.404 |
| Median (range) | 0.755 (0.28–12.07) | 0.5750 (0.281–19.972) | 0.4480 (0.194–6.565) |
Statistics – a–b: p=0.788, b–c: p=0.159, a–c: p=0.220.
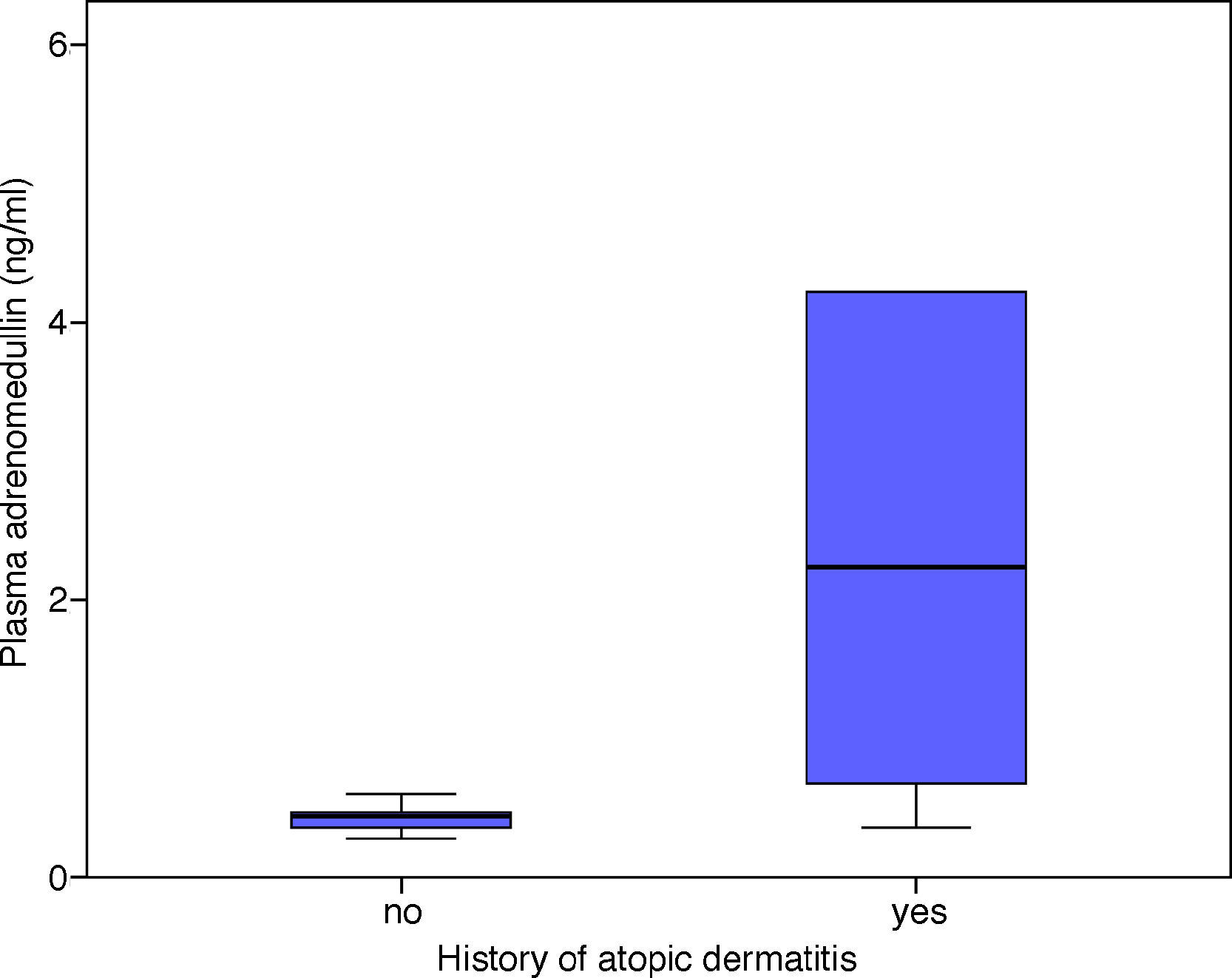
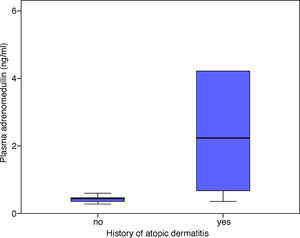
There was a statistically significant correlation between the patients’ AlaTOP levels and plasma ADM levels in the acute attack period (r=0.489, p=0.010). A similar significant correlation was detected between the patients’ Food Panel 7 levels and plasma ADM levels in the acute attack period (r=0.583, p=0.001). No significant correlation was found between the allergy skin test positivity and ADM levels in the acute attack period (p=0.145). The ADM levels in patients with a history of atopic dermatitis were significantly higher in the acute attack period compared to those without a history of atopic dermatitis (p=0.007) (Fig. 2). There was no significant relationship between the ADM levels of asthmatic patients, which were measured at remission period, and patients’ history of atopic dermatitis (p=0.386).
There was no correlation between the forced expiratory volume in one second (FEV1) values in the pulmonary function test and ADM levels in either the acute attack (p=0.362, r=0.222) or remission period (p=0.998, r=0.004).
DiscussionIn the present study, we did not detect any significant difference between the ADM levels in the acute asthma attack and remission periods, whereas Kohno et al. reported elevated ADM levels in the acute attack period compared to remission in adult patients.7 A study by Ceyhan et al., which was conducted in Turkey, did not report any difference in ADM levels between patients with asthma, and healthy individuals.10 Furthermore, the same study found a negative correlation between FEV1 and ADM levels.10 However, we did not find such a correlation in our study. On the other hand, our study demonstrated a significant increase in ADM levels in patients who were considered as having a “severe acute asthma attack period”, when compared to the remission period. We suggest that intensive inflammation during severe asthma attack may increase the release of ADM from bronchial cells.
It has been shown that calcitonin gene-related peptide (CGRP) and ADM are present in sensory nerve fibre keratinocytes, melanocytes and fibroblasts in the skin, and induce vasodilatation and oedema formation, while also enhancing inflammatory factor-induced plasma extravasation.11–13 Some studies stressed the vascular actions of ADM in mammalian skin, such as oedema formation and vasodilation.14,15 ADM also regulates cell signalling in HaCaT cells.14,16 All of these researches have demonstrated that ADM has some impact on skin function and allergy.
It has also been shown that some inflammatory diseases in childhood, including Henoch–Schönlein purpura, familial Mediterranean fever, and acute rheumatic fever, are associated with significantly higher ADM levels during the acute phase.17–19
Our study showed that the patients with a history of atopic dermatitis had significantly higher ADM levels during the acute asthma period as compared to other children. In addition, we detected a significant correlation between ADM levels during the asthma attack period and AlaTOP and Food Panel 7 levels in children with asthma. The observed increased ADM levels in the asthmatic patients with a history of atopic dermatitis might be related to different mechanisms of action in the pathogenesis of immune response in asthma. Mainly, cellular immune response plays a role in atopic dermatitis, while natural immune and acquired immune responses accompany.20 Recently, it has been shown that deficiency in filaggrin, which is an epidermal barrier protein, has been correlated with the pathogenesis of atopic dermatitis.21 Cytotoxic T cell count is reduced in atopic dermatitis,22 and while Th2 cytokines mainly play a role in asthma, Th1 and Th2 together play a role in atopic dermatitis.23 Previous studies have shown that the history of atopic dermatitis is a risk factor for persistent asthma.24–26 According to our experience, individuals with a history of atopic dermatitis have significantly higher Food Panel 7 and AlaTOP levels (unpublished data). Considering the intensive inflammation during acute asthma attacks in patients with a history of atopic dermatitis,25,27 the correlation between Food Panel 7 and AlaTOP levels and ADM in the present study may show the increased stimulation of ADM in these patients.
Many studies have shown the anti-inflammatory nature of ADM,28,29 and its increase in some inflammatory diseases has been attributed to compensation. However, experimental studies13,15 claim that it may contribute to the development of inflammatory oedema. In atopic dermatitis, an increase in ADM levels may be expected due to elevated excitability of the skin against the inflammation. However, considering the design of our study, it was not possible to evaluate which cells secreted ADM or whether ADM had inflammatory or anti-inflammatory effects in these patients.
This study demonstrated that ADM may have a role in the immuno-inflammatory process of asthma, although whether it acts to preserve or protect against further inflammatory injury is not clear. Considering the positive correlation between the plasma levels of AlaTOP/Food Panel 7 and ADM levels, we also speculate that ADM may have a role in children with atopic dermatitis. However, the exact role of ADM in the pathogenesis of asthma and atopic dermatitis requires further, detailed investigations.
FundingThis study was not financially supported by any foundation or company.
Conflict of interestThe authors report no competing interest. The authors alone are responsible for the content and writing of this paper.