Perioperative anaphylactic reactions are immediate, hypersensitive reactions that are potentially life-threatening resulting from a sudden release of mediators from mast cells and basophiles, due to either immune (IgE or non-IgE mediated) or non-immune mechanisms. The most frequent causing agents are neuromuscular blocking agents (NMBAs), latex and antibiotics, with latex being the first cause in paediatrics. With regard to perioperative anaphylactic reactions, the usual early signs and symptoms of an anaphylactic reaction could be overlooked or erroneously interpreted and non-severe anaphylaxis could go undetected, with a risk of more severe reactions in the future. Using the data registered on the anaesthesia sheet, it is essential to establish a chronological relationship between drugs and/or substances administered and the reaction observed. An elevated level of tryptase confirms an anaphylactic reaction, but this does not usually increase in the absence of compromised circulation. An allergy study should be carried out preferably between 4 and 6 weeks after the reaction, using a combination of specific IgE, skin and controlled exposure tests (if indicated). Test sensitivity is good for NMBAs, latex, antibiotics, chlorhexidine, gelatine and povidone, and poor for barbiturates, opiates (these can give false positives since they are histamine releasers) and benzodiazepines. Special preventive measures should be taken, especially in the case of latex. We present the maximum concentrations recommended for skin tests, the recommended dosage to treat anaphylactic reactions in paediatrics and a procedure algorithm for the allergological study of these reactions.
Perioperative anaphylactic reactions are potentially life-threatening, immediate, hypersensitive reactions. The European Academy of Allergy and Clinical Immunology (EAACI) and the World Health Organization propose that these anaphylactic reactions be classified as allergic anaphylaxis (immune) rather than non-allergic (known to date as anaphylactoid or pseudoanaphylactic).1 All these are a result of the release of pre-formed and newly synthesized mediators from the mast cells and basophiles.
Many of the drugs or substances used in perianaesthesia can provoke adverse reactions related to their pharmacological properties (usually dose-dependent), or unrelated to the same (less dose-dependent), with the latter corresponding to: intolerance, idiosyncratic and anaphylactic reactions (immune or non-immune).
PrevalenceThe estimated incidence of perioperative anaphylactic reactions in Spain is one in every 10,263,2 and in other countries this varies widely between 1/1700 and 1/20,000.3 In many of these series, immune reactions represent 60% of all hypersensitive reactions observed during the perioperative period, with a mortality rate of between 3 and 9%.
MechanismAnaphylaxis is a potentially lethal, acute, multi-systemic syndrome, resulting from the sudden release of mediators into the circulation by mast cells and basophiles.4 Perianaesthetic anaphylaxis can be produced by the following mechanisms1:
- -
IgE mediated mechanism, which represents approximately 60% of cases.
- -
Non-IgE mediated, immunologically caused (non-IgE mediated immunological anaphylaxis, formerly known as anaphylactoid reactions), with reactions mediated by IgG or IgM being included in this category, or by antigen-antibody plus complement complexes.
- -
Non-immunological direct release of histamine and other mediators from mast cells and basophiles.
Allergic anaphylaxis is caused by the interaction of an allergen with IgE. In sensitised individuals, these antibodies are bound to high affinity receptors in mast cells and basophiles and to low affinity receptors in lymphocytes, eosinophiles and platelets. Pre-formed and newly synthesized mediators are released such as: histamine, tryptase, PG2, leukotrienes, thromboxane A2, platelet activating factor, chemokines and cytokines, which explain the clinical findings.5 Allergic anaphylaxis to some substances such as dextrans can be caused by IgG immunocomplexes with the antigen, which activate the complement's system.
The mechanisms of non-immune reactions are not well established, but they are considered to be a result of the direct stimulation of mast cells and basophiles with mediator release.5
On the other hand, several causing agents such as neuromuscular blocking agents (NMBAs) are capable of causing a reaction via more than one mechanism.6
Whatever the mechanism, the initial management of the reaction is the same, and the clinical severity similar, even though some differences can be appreciated between the different causing mechanisms7:
- -
Only IgE mediated reactions can be studied with skin tests.
- -
The severity of IgE mediated reactions can increase with the subsequent administration of the causing agent, whilst the others remain similar.
- -
The frequency or intensity of non-IgE mediated reactions (e.g. contrast reactions), can be reduced with pre-medication.
- -
Non-IgE mediated reactions do not require previous contact with the substance. This is generally required for IgE mediated reactions, although there are cases where this happens on first contact, which could be due to a cross-reaction with other substances to which the patient is sensitised.
Even though numerous possible causing agents exist, the cause cannot be identified in a significant number of cases. The best data concerning perioperative anaphylaxis come from the series pertaining to a French multi-centre study (started around the mid-1990s and currently on-going).3,8
NMBAs are the most common agents, being the most frequently involved substances, ranging between 50 and 70%, followed by latex (12–16.7%), and in recent reports, by antibiotics (15%).3
Neuromuscular blocking agentsThese can cause anaphylaxis either by IgE-mediated mechanisms or by non-immunological direct activation of the mast cells, with the IgE mediated reactions being the most severe.
Groups of tertiary and quaternary ammonium are the principal components of these drugs’ allergenic sites.3 Crossed-sensitivity is frequent (between 60 and 70% of cases) and this is believed to be due to the shared quaternary ammonium group.9
Cross-reactivity to all is unusual, with this being more frequent between those of the same group, although this can also occur between different groups of NMBAs and can also happen more frequently between NMBAs, aminosteroids (pancuronium, vecuronium, rocuronium), than with benzylisoquinoline (D-tubocurarine, atracurium and mivacurium).3
Suxamethonium (depolarising blocker, succinylcholine), is the most implicated in anaphylactic reactions, followed by atracurium and rocuronium.10 Between 20 and 50% of reactions to relaxants are of non-immune origin, with these generally being less severe than IgE-mediated ones, except in a sub-group of hyper-responders to released histamine and they occur, above all, with benzylisoquinoline derivatives (tubocurarine, atracurium and mivacurium).11
Cases of IgE-mediated anaphylaxis to NMBAs have been reported on first contact with the drug in 15–50% of cases and it is speculated that these cases could be due to a previous sensitisation to similar substances which contain tertiary or quaternary ammonium ions.3 It has been discovered that pholcodine could be one of these agents and other substances as yet unknown could be related due to their possible presence in daily-use products such as: toothpaste, washing detergents, shampoos, etc. IgE to pholcodine can occur in the absence of pholcodine intake which thereby suggests that other environmental factors could produce IgE antibodies which react not only to pholcodine but also to suxamethonium and morphine as well. These antibodies could be an in vitro phenomenon and it has not been possible to demonstrate the causal association between the prevalence of these and the existence of NMBA-induced anaphylaxis.12
LatexThis is the second most common cause of perioperative anaphylaxis. In children who have undergone numerous operations, particularly those with spina bifida,13 it is the first cause of anaphylaxis. In 30% of cases, there were symptoms noted in the patient's history which suggested that these could have occurred previously.14 After a large increase of cases, these have been reduced during the last few years by applying preventive measures.
AntibioticsThese are frequently administered perioperatively. β-Lactams cause 70% of perioperative reactions to antibiotics and represent between 12 and 15% of perioperative reactions.14Vancomycin has been incriminated in some cases, although the majority of these cases correspond to non-immune reactions (Red Man Syndrome), being rare allergic reactions.15Quinolones are the third cause of reactions to antibiotics (except in paediatrics),16 and bacitracin and rifampicin used to flush wounds, can potentially cause anaphylactic reactions.3
AnalgesicsIn Spain, these appear in some series, including in up to 14–27% of perioperative reactions (if we also consider the post-operative reanimation period).2 This is principally due to the frequent use of metamizole (the most sensitising pyrazolone) in Spain compared to in other countries.2
Hypnotics (inductors)These are responsible for approximately 2% of perioperative anaphylactic reactions.8 They are divided into two types: barbiturates and non-barbiturates.
- -
Barbiturates: Thiopental must be highlighted, whose incidence has been estimated at around 1/30,000, and in less than 1% of perioperative reactions in the French series.8 It is widely used in anaesthesia and can cause almost all reactions within the group of inducting agents. Many reactions are IgE mediated. The female population is three times more affected.
- -
Non-barbiturates: Benzodiazepines (midazolam), propofol, etomidate, ketamine and inhaled anaesthetics. Allergic reactions to these drugs are relatively rare, and many of the generalised reactions are believed to be related to their ability to directly release histamine, although IgE-mediated reactions have been evidenced through skin and specific IgE tests.
- -
Reactions caused by propofol account for less than 2% of perianaesthetic reactions.14 With respect to the rest of hypnotics, reactions are rarer and no reactions of immediate hypersensitivity to inhaled anaesthetics have been reported.14
Allergic reactions to morphine, codeine, meperidine, fentanyl and derivatives are rare. Due to their histamine-releasing properties (particularly morphine), the distinction between anaphylaxis and the release of non-immune mediators is not always easy.14
Opiates typically cause non-IgE mediated skin symptoms since the drug binds to opiate receptors in mast cells, thereby releasing histamine and other mediators which cause erythema, urticaria and angio-oedema.5 IgE reactions to morphine or fentanyl have been implied in some cases, even though skin tests are not valid for these drugs.17
Local anaestheticsThese include the amino group and the ester derivatives of benzoic acid. Reactions are rare and less than 1% of them have an allergic mechanism.18 The most common cause is inadvertent intravascular injection, or systemic absorption of epinephrine combined with local anaesthetic. The para-amino group (procaine, tetracaine, etc.), has a greater ability to cause allergic reactions than the amide group (lidocaine, bupivacaine, mepivacaine, etc.). They are involved in less than 0.6% of perioperative reactions.14 The reactions can also be caused by preservatives in commercial presentations (methylparaben, parabens, or metabisulfite).14
ColloidsAll synthetic colloids have been shown to cause anaphylactic reactions. They are responsible for about 4% of all perioperative anaphylactic reactions,19 although the incidence is decreasing due to the increasingly widespread use of hydroxyethyl starch solutions (Voluven).
Dextrans and gelatines are more commonly incriminated than albumin or others (hydroxyethyl starch). Reactions to gelatine are responsible for 95% of colloid reactions.19 These may be due to direct histamine release (especially gelatine bound to urea), or IgE mediated.
Adverse reactions with gelatine bound to urea are more common (0.85%) than those with modified fluid gelatine (succinate-linked) (0.33%).20 Gelatine is present in plasma expanders as gelatine bound to urea (Haemaccel), and as succinylated gelatine (Gelofusine).
Colorants (dyes)These are a rare cause of anaphylaxis. Many cases of hypersensitivity to blue dyes (blue isosulfan) have been reported,21 and it is believed that sensitisation is caused by the daily use of products which contain these colorants. An increase is expected in the number of cases, given the increased use in lymph duct mapping for sentinel node biopsies.
Reactions can be IgE mediated and/or from the direct release of mediators. Methylene blue does not have a cross-reaction with other blue dyes, and anaphylactic reactions to the same would appear to be rare.22 Reactions to dyes are difficult to diagnose since they are relatively late starting (30min after injection), long lasting, intense and severe.22
Other agentsThis group of diverse medications and agents cause less than 5% of perioperative anaphylactic episodes.7 Anaphylactic reactions caused by chlorhexidine can occur after the insertion of central catheters soaked in the same, or from its intraurethral use or in gynaecological procedures or topical application.23 Rare cases of anaphylaxis caused by the topical use of povidone have been reported.14 In both cases, skin tests can provide a diagnosis.
Protamine used to reverse heparin anticoagulation can lead to reactions mediated by IgE, IgG or complement, and the incidence of perioperative anaphylaxis varies between 0.19 and 0.69%.24
Reactions may also occur with: radiographic contrast agents, blood transfusions in cases of severe IgA deficiency, metabisulfites and sulphites in medications, NSAIDs, bacitracin in irrigation solutions, ethylene oxide used as a steriliser, streptokinase and urokinase, chymopapain, insulin, heparin and other anticoagulants, hyaluronidase and neostigmine.7
Clinical manifestationsThe initial diagnosis of anaphylaxis is based on presumption, although such diagnosis is essential since anaphylaxis advances in minutes and can be life threatening. Signs of anaphylaxis during anaesthesia differ somewhat from the signs and symptoms that occur during anaphylaxis not associated to the same.
Early signs usually observed in patients who are awake are absent when they are anaesthetised.
Skin symptoms can be difficult to appreciate in patients completely covered up and many signs such as increased heart rate, decrease in blood pressure, or an increase in airways resistance, could initially be misinterpreted as a result of the interaction between the clinical state of the patient and the drugs administered during the procedure (dose-related effects), or to an excessively light anaesthetic.3
For these reasons, many grade I and II anaphylaxis go undetected,25 with the subsequent risk of more severe reactions during future anaesthesia. Reactions vary between slight hypersensitive reactions to severe anaphylactic shock, or even death.3
IgE-mediated reactions are usually more severe than non-IgE mediated ones,14 even though the signs of both cases can be very similar.25,26 Reactions to NMBAs have been seen to be more severe than those to latex.14
Anaphylaxis can occur at any time during anaesthesia, appearing after just a few seconds or up to 60min from administration,7 and can progress quickly or slowly.3
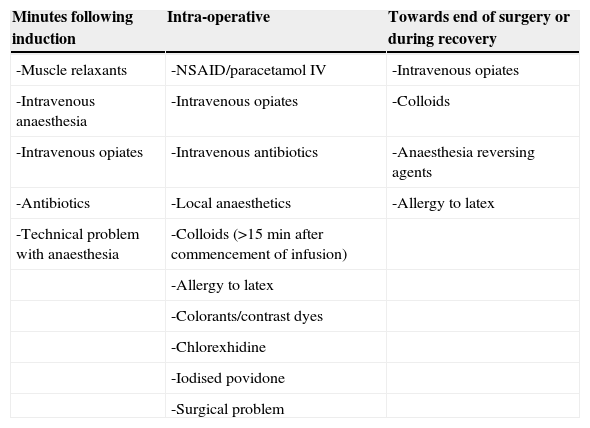
Cases occur 90% of the time during anaesthetic induction during the minutes following intravenous injection of the offending agent, as with NMBAs, antibiotics or inductors.26 If signs appear during anaesthetic maintenance then this suggests allergy to latex, volume expanders, antibiotics or dyes3 (Table 1).
Commencement of adverse events corresponding to the causing agent.
| Minutes following induction | Intra-operative | Towards end of surgery or during recovery |
|---|---|---|
| -Muscle relaxants | -NSAID/paracetamol IV | -Intravenous opiates |
| -Intravenous anaesthesia | -Intravenous opiates | -Colloids |
| -Intravenous opiates | -Intravenous antibiotics | -Anaesthesia reversing agents |
| -Antibiotics | -Local anaesthetics | -Allergy to latex |
| -Technical problem with anaesthesia | -Colloids (>15min after commencement of infusion) | |
| -Allergy to latex | ||
| -Colorants/contrast dyes | ||
| -Chlorexhidine | ||
| -Iodised povidone | ||
| -Surgical problem |
The initial clinical findings frequently observed by anaesthetists are: decrease or loss of pulse, lowering of blood pressure, difficulty with lung inflation and generalised erythema.27 Skin symptoms have been observed in 66–70% of patients with IgE-mediated reactions and in more than 90% of non-IgE mediated reactions.14 To the contrary, cardiovascular collapse and bronchospasm are observed in between 39 and 50% of IgE mediated reactions, and only in between 11 and 19% in non-IgE mediated ones.14
The absence of skin symptoms does not exclude a diagnosis of anaphylaxis,3 and even sudden cardiac arrest can occur without any other clinical symptoms.14
A reaction with only one clinical detail (bronchospasm, tachycardia with hypotension) can easily be missed, since many other pathological conditions can have an identical presentation, and the absence of a diagnosis in these cases can give way to a fatal re-exposure.3
In some cases the bronchospasm can be particularly severe and resistant to treatment, with the risk of cerebral anoxia or death.3 Asthmatics have a special risk of suffering significant bronchospasm within an anaphylactic reaction.28
To evaluate the severity of perioperative anaphylaxis, the classification recommended by Mertes and Laxenaire26 can be used (see Table 2).
Severity of immediate hypersensitivity.
| Grade | Symptoms |
|---|---|
| I | Skin symptoms: Generalised erythema, urticaria, and angio-oedema. |
| II | Non-life threatening measurable signs: Skin symptoms, hypotension, tachycardia, and respiratory disorder: cough, breathing difficulties. |
| III | Serious life-threatening symptoms: Collapse, tachycardia or bradycardia, arrhythmias, bronchospasm. |
| IV | Cardiac and/or respiratory arrest. |
Special mention should be made of data collected in the French study of anaphylaxis during anaesthesia,8 relating to the characteristics of the subgroup of 266 children under 18 years.
Of these, 122 were diagnosed with IgE-mediated reactions. Incriminated substances differ substantially from those of adults. Latex was the most frequent cause (41.8% of cases), being diagnosed as from 2 years old, whilst NMBAs (31.97% of cases), were the most frequent cause in adolescents. In contrast to adults, no significant differences were observed in gender distribution for both mechanisms (IgE mediated or non-IgE mediated).
Just like adults, no differences were observed between IgE-mediated and non-IgE mediated reactions as regards to atopy, asthma, or drug intolerance. However, a larger number of cases of IgE-mediated reactions in atopic patients sensitised to latex were seen and also in the history of food allergies, compared to adults.
Clinical manifestations were more severe with IgE-mediated reactions, many being grade 3, whilst non-IgE mediated reactions were only grade 1. The cardiovascular symptoms were more frequent in IgE-mediated reactions than in non-IgE mediated ones. Only one case of anaphylactic shock was reported. Cases of bronchospasm were also more frequent in IgE-mediated reactions.
Study of the perioperative allergic reactionAny suspected hypersensitive reaction during anaesthesia must be extensively investigated by pre and post-operative tests.3
The responsible drug must be identified as well as possible cross-reactivity in the case of muscle relaxants, and recommendations should be given for future anaesthesias.3
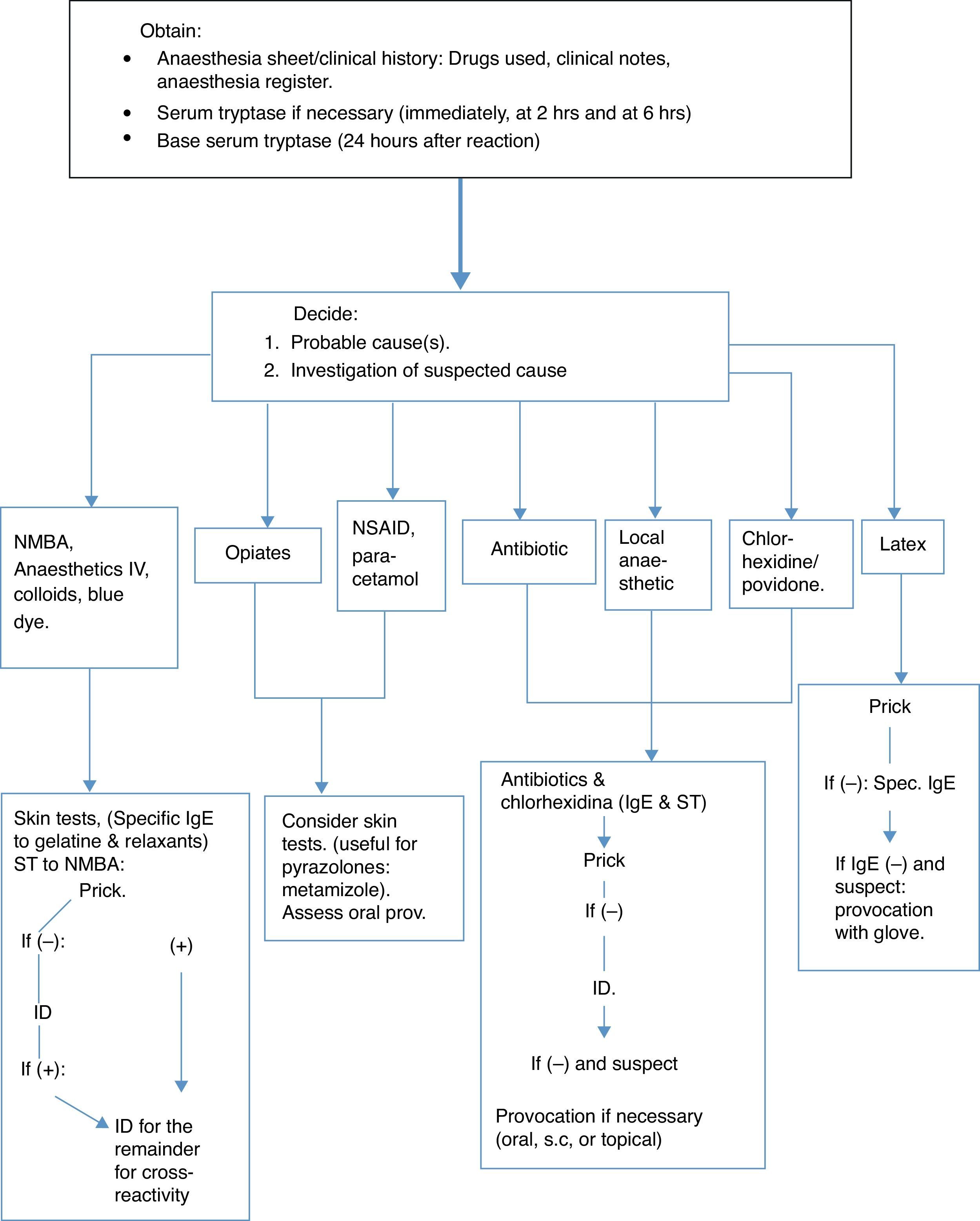
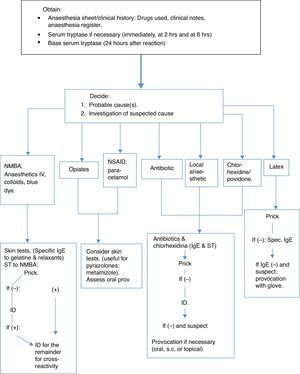
The diagnostic strategy is based on a detailed history of the reaction, including concurrent morbidity, previous anaesthetic history and known allergies, together with a combination of investigations carried out: some at the time of the reaction (mediators released, especially tryptase, to see if the mast cells are activated); others, such as specific IgE can be determined immediately but are recommended 4–6 weeks afterwards, without leaving it later than the first 6 months. Skin tests can be carried out during the year following the event, but not immediately afterwards (after 4–6 weeks), and other tests can be made such as the histamine release or basophile activation tests.3 See the algorithm based on the Guidelines published by the British Society for Allergy and Clinical Immunology (BSACI)11 (Fig. 1).
It is essential to have the previous history. For example, in an asthmatic patient, bronchospasm could be the only symptom of an anaphylactic reaction, but this can also occur in the absence of the same.11
It is obligatory to keep a registry of drugs as they are administered, and these must be clearly reflected on the anaesthesia sheet.11 It is essential to establish at which stage of anaesthesia a reaction occurs.11 (Table 1).
Anaphylaxis to induction agents, NMBAs and antibiotics generally occur a few minutes after administration, since a large bolus of allergen is intravenously administered.
To the contrary, as regards allergy to latex, the allergen is absorbed more slowly from the surgeon's gloves, through the peritoneum, mucosa or skin and therefore the onset is slower and occurs intra-operatively, maybe up to 30min after the initial contact with latex, although this interval would depend upon the patient's sensitivity, the amount of allergen absorbed, and the contact surface (it is more quickly absorbed through the vagina or peritoneum than through the skin).
A reaction to gelatine usually occurs around 15–20min after starting the infusion (colloids). Reactions to chlorhexidine can be delayed by 10min after contact with the mucosa (such as bladder instillation) and later, in the case of skin antisepsis, even though direct entry into the circulation via the insertion of a central venous catheter can result in immediate circulatory collapse.11
By perusing the clinical history, we can establish whether the patient is part of the risk population,3 if:
- -
The patient is allergic to one of the drugs or products that are likely to be administered during the procedure.
- -
The patient showed signs that suggest an allergic reaction during previous anaesthesia.
- -
The patient has had allergic clinical symptoms upon exposure to latex.
- -
The patient is a child undergoing multiple operations, especially those with spina bifida, due to the high frequency of sensitisation and anaphylactic shock to latex in these kinds of patients.
- -
The patient is allergic to avocado, kiwi, banana, chestnut, pineapple, etc., due to the high frequency of cross-reaction of these elements with latex.
During an IgE-mediated reaction, the basophiles and mast cells activate and de-granulate, releasing mediators which can be measured in patient serum and can be useful for the diagnosis of anaphylaxis during anaesthesia.3
Tryptase in serum is specifically elevated by the activation of mast cells and is released both in IgE-mediated anaphylactic reactions as well as non-IgE mediated ones,11 even though a clearly elevated concentration of above 25mcg/ml would suggest an IgE-mediated anaphylaxis.29
Tryptase reaches a peak after 30min and high levels can still be seen up to 6h or more, later.3 Serial samples are recommended: Immediately; at 2 and 6h; and another after 24h to obtain the patient's baseline value and rule out mastocytosis (above 20mcg/ml), which carries a serious risk of surgical anaphylaxis.11
However, the absence of an elevated tryptase level does not rule out an anaphylactic reaction, since this does not usually rise in the absence of shock or hypotension.30 To diagnose anaphylaxis the tryptase has 64% sensitivity and 89% specificity, with a VPP of 92.6% and VPN of 54.3%.30
Specific IgE serumThis may be performed for suxamethonium, thiopental, gelatine, antibiotics and latex11 and also: chlorhexidine, chymopapain, protamine, ethylene oxide and morphine, all of which are commercially available. It is also possible for propofol and rocuronium.3 The sensitivity of these IgE assays is variable. ImmunoCAP for rocuronium has a sensitivity of more than 85% and almost 100% specificity.31
With latex, sensitivity is up to 92%3; IgE to antibiotics shows less sensitivity than skin tests (0–75%)3,32; and with respect to suxamethonium, this is between 30 and 60%.10 Radioimmunoassay with morphine to detect specific IgE to muscle relaxants has a greater sensitivity (85%) and very high specificity (98%).33
Given the possibility of a false negative result due to the consumption of IgE during the reaction, it is recommended to carry out this test several weeks later, although it is also valid to carry out the same during the reaction or immediately afterwards, since a positive result is not excluded at that moment.25
Skin testsPrick and intradermal tests are generally more sensitive than in vitro techniques.10 For the same reasons as with specific IgE, these tests are carried out between 4 and 6 weeks after the reaction,34 although they can be carried out beforehand.11 The drugs used for anaesthetic induction suspected of causing an anaphylactic reaction, cannot be re-administered and therefore positive tests can never be totally valid.11 Some drugs (opiates, atracurium, and mivacurium) have direct histamine release activity and can cause false positive results in normal subjects.11 This fact must be taken into special consideration regarding opiates.35
Dilutions are prepared from the commercial presentation.3,11 There is no complete general consensus regarding the concentrations for prick and intradermal, or even the interpretation of these tests.3,11
The recommendation in the UK is that the PRICK test to anaesthetic agents should always be carried out using two concentrations: undiluted and diluted at 1/10 to reduce false positives caused by histamine-releasing activity,11 which is not considered in the French study.3
Everybody coincides regarding the use of a negative (saline) and positive (histamine) control. There is a consensus regarding the interpretation of skin prick tests. After 15–20min, this is considered positive if the wheal is at least 3mm greater than the negative control. With respect to intradermal tests however, some consider an increase in the papule of twice or more the size of the initial papule, to be positive,11 and others, an increase of 3mm with erythema (as a standard).3
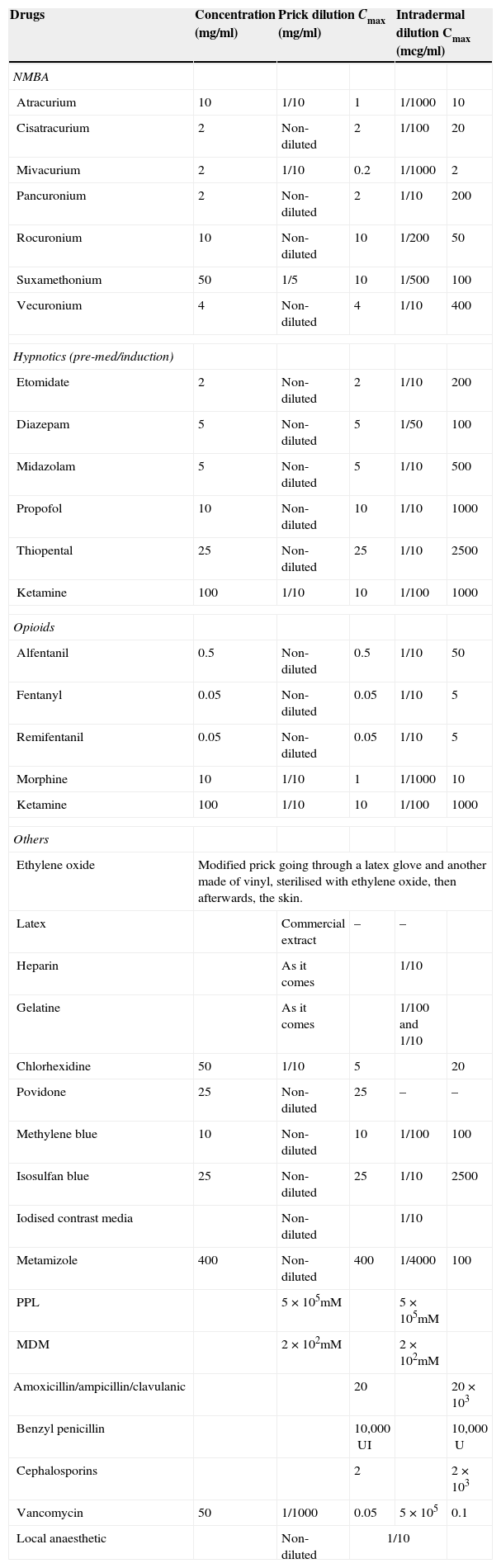
Given the considerable experience accumulated by the French study, concentrations are recommended by the SFAR and the French Society of Allergology for skin testing for drugs used in anaesthesia3 as well as some changes proposed by the Allergy to Drugs Study Group ENDA/EAACI,36 with further substances of interest being added (gelatine, latex and ethylene oxide, povidone and metamizol) (Table 3).
Non-irritant concentrations recommended for skin tests.
| Drugs | Concentration (mg/ml) | Prick dilution Cmax (mg/ml) | Intradermal dilution Cmax (mcg/ml) | ||
|---|---|---|---|---|---|
| NMBA | |||||
| Atracurium | 10 | 1/10 | 1 | 1/1000 | 10 |
| Cisatracurium | 2 | Non-diluted | 2 | 1/100 | 20 |
| Mivacurium | 2 | 1/10 | 0.2 | 1/1000 | 2 |
| Pancuronium | 2 | Non-diluted | 2 | 1/10 | 200 |
| Rocuronium | 10 | Non-diluted | 10 | 1/200 | 50 |
| Suxamethonium | 50 | 1/5 | 10 | 1/500 | 100 |
| Vecuronium | 4 | Non-diluted | 4 | 1/10 | 400 |
| Hypnotics (pre-med/induction) | |||||
| Etomidate | 2 | Non-diluted | 2 | 1/10 | 200 |
| Diazepam | 5 | Non-diluted | 5 | 1/50 | 100 |
| Midazolam | 5 | Non-diluted | 5 | 1/10 | 500 |
| Propofol | 10 | Non-diluted | 10 | 1/10 | 1000 |
| Thiopental | 25 | Non-diluted | 25 | 1/10 | 2500 |
| Ketamine | 100 | 1/10 | 10 | 1/100 | 1000 |
| Opioids | |||||
| Alfentanil | 0.5 | Non-diluted | 0.5 | 1/10 | 50 |
| Fentanyl | 0.05 | Non-diluted | 0.05 | 1/10 | 5 |
| Remifentanil | 0.05 | Non-diluted | 0.05 | 1/10 | 5 |
| Morphine | 10 | 1/10 | 1 | 1/1000 | 10 |
| Ketamine | 100 | 1/10 | 10 | 1/100 | 1000 |
| Others | |||||
| Ethylene oxide | Modified prick going through a latex glove and another made of vinyl, sterilised with ethylene oxide, then afterwards, the skin. | ||||
| Latex | Commercial extract | – | – | ||
| Heparin | As it comes | 1/10 | |||
| Gelatine | As it comes | 1/100 and 1/10 | |||
| Chlorhexidine | 50 | 1/10 | 5 | 20 | |
| Povidone | 25 | Non-diluted | 25 | – | – |
| Methylene blue | 10 | Non-diluted | 10 | 1/100 | 100 |
| Isosulfan blue | 25 | Non-diluted | 25 | 1/10 | 2500 |
| Iodised contrast media | Non-diluted | 1/10 | |||
| Metamizole | 400 | Non-diluted | 400 | 1/4000 | 100 |
| PPL | 5×105mM | 5×105mM | |||
| MDM | 2×102mM | 2×102mM | |||
| Amoxicillin/ampicillin/clavulanic | 20 | 20×103 | |||
| Benzyl penicillin | 10,000UI | 10,000U | |||
| Cephalosporins | 2 | 2×103 | |||
| Vancomycin | 50 | 1/1000 | 0.05 | 5×105 | 0.1 |
| Local anaesthetic | Non-diluted | 1/10 | |||
Suggested changes to maximum concentrations for ID (36): Mivacurium 1/200: 10mcg/ml. Suxamethonium 1/100: 500mcg/ml. Vecuronium 1/100: 40mcg/ml. Pancuronium 1/50: 40mcg/ml.
Skin tests to NMBAs can remain positive, years after a reaction,3 whilst those for β-lactams decrease with time.
Due to the frequent cross-reactivity between NMBAs, all these must be tested and only the ones with negative results can be re-used again in the future.3,11 The predictive value of these tests is generally sufficient, although some isolated cases exist of later reactions after negative results.37 The sensitivity of skin tests to NMBAs is between 94 and 97%.38 It is good for synthetic gelatines and β-lactams, and poor for barbiturates, opiates and benzodiazepines.39
Although there has been some controversy regarding the benefits of the prick test compared to intradermal tests, both techniques however, are still recommended.3 The prick test is more useful to identify the NMBA responsible for the anaphylactic reaction, and the intradermal to investigate cross-reactions with other NMBAs.40Latex sensitisation is investigated by prick test because of its high sensitivity and specificity.41
Both prick and intradermal tests are suggested for blue dyes, with the latter being recommended at a dilution of 1/100.22 Intradermal tests have given positive results with colloid gelatines, but negative using the prick test.11 Both intradermal and prick tests are useful with chlorhexidine,42 and the prick test for povidone is very sensitive.43,44
Mediator release testThe histamine and sulfidoleukotrienes release tests and flow cytometry are not very sensitive. They are complex and have to be better validated and are not currently considered useful in practice.3
In certain cases however, such as in NMBA allergic patients identified by elevated tryptase and IgE to morphine but with negative skin tests, the basophile activation test might be the only way to confirm the diagnosis and to identify a safe alternative.45
Controlled exposure testThe indications for these tests are limited and are restricted to local anaesthetics, β-lactams, NSAIDs and latex, and are only carried out after negative skin tests.3
PreventionPrevention of sensitisationAllergy to latex in children with spina bifida or undergoing multi-surgery can be prevented by avoiding the use of latex and this strategy has proven to be effective.46 The use of low-content latex gloves amongst health workers has been proposed as a measure for reducing the level of latex aeroallergens.3
Avoid the causing agentThe prevention of anaphylactic reactions principally depends upon a detailed documentation of previous reactions. To carry this out, a detailed history of atopy during the pre-anaesthesia consultation must be obtained and any allergies to drugs, latex and tropical fruits must be recorded.
- -
During emergency operations when any previous reactions are unknown, regional anaesthesia is recommended and if possible, a latex free operating room.47 If general anaesthetic is required, volatile anaesthetics should be used since allergy to these has not been described.3,10
- -
If allergy to NMBAs is suspected, it is important to generally avoid using others from the same group due to frequent cross-reactivity.
- -
Gelatine solutions must be avoided in allergy to vaccines containing gelatines, recommending the use of hydroxyethyl starch (Voluven), or serum albumin instead.
- -
No reactions to propofol have been proven in subjects allergic to egg48 (which was a possibility suggested by the presence of egg lecithin in its composition), although isolated and rare cases cannot allow us to completely exclude this possibility, and prudence should be exercised in cases of anaphylaxis.49
- -
Allergy to latex requires complete avoidance of the same. These kinds of patients should be the first to be operated on to thereby avoid exposure to aerosolised particles and they should also wear a medical alert bracelet. Signs should be hung both inside and outside the operating theatre and obviously, only totally latex-free materials should be used.3,47
Pre-treatment with corticosteroids and antihistamines, either anti-H1 or combined anti-H1 and H2 antagonists, remains controversial.3,15 Pre-treatment has shown a reduction in the severity but not the incidence of adverse reactions to dyes, and has generally proven effective to minimise the adverse effects of non-immune release to: NMBAs, gelatine, contrasts and vancomycin.3
No evidence exists regarding the beneficial effects of pre-treatment with corticosteroids regarding allergic reactions to anaesthetic drugs. However, some allergic reactions to anaesthetic drugs after preoperative preventive treatment have been recorded, but it is generally considered that pre-treatment with corticoids and antihistamines does not prevent immune-type reactions.50
TreatmentDuring anaesthesia, the patient must be monitored and have good intravenous access. When faced with an anaphylactic reaction, all contact with the antigen responsible must be interrupted, as well as the drugs that are being administered, unless this is impossible. Airways will be maintained with 100% oxygen and the recommended treatment for anaphylaxis carried out (see Table 4).16,25,51
Treatment of perioperative anaphylactic reactions in paediatrics.
| In light of a suspected anaphylactic reaction | Inform the surgeon. Immediately request help. Stop all drugs, colloids, haematic products, etc., and latex (if suspected) |
| Airways | Airways with 100% O2 |
| Epinephrine | IM: 0.01mg/kg/dose: >12 years: up to 0.5ml (500mcg) (at 1/1000); <12 years (up to 0.3ml).This can be repeated. If insufficient, proceed with an IV infusion: 1mg adrenaline+100ml of SSF: 0.01mg/ml: 1/100,000; 1ml/kg/h: 0.01mg/kg/h (0.17mcg/kg/min).Initial dose: 0.1mcg/kg/min, up to 1mcg/kg/min (maximum up to 2–5mcg) |
| Fluid therapy | Crystalloid (SSF): 20ml/kg in 20min or colloid (serum albumin 5% or Voluven): 10ml/kg in 20min. If necessary, this can be repeated |
| Anaphylaxis resistant to epinephrine | |
| Glucagon | 20 a 30μg×kg (up to 1mg) |
| Norepinephrine | 1mg+100ml of glucose serum: 0.01mg/ml.Start: 0.05 at 1mcg/kg/min |
| other supporting drugs | |
| Atropine | 0.02mg/kg |
| Dopamine | 3mg×weight in kg: mg of dopamine to be diluted in 50ml of serum.Dose: 5–20mcg/kg/min 1ml/h: 1mcg/kg/min |
| Second line treatments | |
| Salbutamol/ipratropium | -Salbutamol: MDI: 4 puffs every 10min if necessary, or nebulised 0.03ml/kg/dose (up to 1ml maximum), every 20–30min-500mcg (0.5mg) of Ipratropium can be added-Salbutamol IV: 5–25μg/min |
| Anti H1 (dexchlorpheniramine) | 0.15–0.3mg/kg/dose slow IV or IM (maximum 5mg) |
| Anti H2 (ranitidine) | 2–8mg/kg diluted IV infusion in 20min |
| Corticosteroids (hydrocortisone) | 10–15mg/kg every 6h |
The paediatrician and/or anaesthetist should identify children at risk prior to surgery, to prevent any possible reactions:
- 1.
Latex should not be used for surgery on children with spina bifida or undergoing multiple surgeries, to prevent sensitistion to the same.
- 2.
Allergy to latex should be ruled out in children allergic above all, to kiwi, banana, avocado, and chestnut.
- 3.
In children known to be allergic to latex, the environment should be totally latex-free.
- 4.
If previous perioperative reactions exist, either to drugs or substances that could be used, for example:
- •
Suspected allergy to NMBAs: Avoid those from the same group and if previous studies exist, only use those that gave a negative result in tests carried out.
- •
Avoid gelatine solutions in patients allergic to gelatine in vaccines, and instead use hydroxyethyl starch (Voluven) or serum albumin.
- •
- 5.
Avoid any drug or substance that caused or was suspected of causing previous reactions, as well as those which may have possible cross-reactivity.
- 6.
Propofol can be used for patients allergic to egg, except for anaphylactic subjects (due to an unlikely, but possible, reaction to the lecithin contained in the drug).
- 7.
It is advisable to use regional anaesthesia if possible, for emergency surgery since the risk factors and previous medical history are unknown. A latex-free operating theatre should be used and volatile anaesthesias are recommended, since allergy to these has not been described.
- 8.
Mastocytosis should be ruled out in patients who have experienced severe or atypical reactions during previous surgery and have a negative allergological study. The use of tourniquets should be avoided in patients with known cases of this illness (due to the release of mediators by mast cells exposed to ischemia).
- 9.
Special care needs to be taken with asthmatic children who are at greater risk of suffering bronchospasm during anaesthesia, which would be extremely severe in the event of anaphylaxis. It is advisable to avoid the use of salbutamol at least 6h before anaesthesia if halogenated anaesthesias are to be used, to avoid the potential risk of arrhythmias or hypotension (Agencia Española de Medicamentos y Productos Sanitarios – Spanish Medicines and Health Products Agency).52
Note down in detail the medication and all substances administered, the doses, and time of administration as well as the time, description and duration of suspicious clinical symptoms and the treatment carried out.
In the event of a suspected anaphylactic reactionIntensive monitorisation.
Adequate ventilation and oxygenation.
Interrupt the administration of the drug, substance or solution.
Treatment for anaphylaxis: adrenaline, fluid therapy and the remainder of the treatment (see Table 4).
Immediately, then after 2, 6, and 24h (base value). Use a dry tube or one with coagulant. The sample remains stable for up to 1 week (2–8°C), or centrifuged and frozen at −20°C.
Specific IgE tests can be carried out, although these can give a false negative result.
Allergological diagnostic studyClinical historyRisk factors, background and anaesthesia sheet (substances and drugs received and chronological relationship of symptoms from the same), for orientation towards the probable causing agent. It should be taken into account that the majority of reactions in children are principally due to latex, followed by muscle relaxants and antibiotics.
Specific IgEWhen suspected, this will be carried out for: suxamethonium, thiopental, bovine gelatine, antibiotics, latex, chlorhexidine, chymopapain, protamine, ethylene oxide and morphine (the latter can also be used to detect sensitisation to NMBAs in general). This can also be requested for rocuronium and propofol. This test should preferably be carried out between 4 and 6 weeks after an incident, but no later than after 6 months.
Skin testsThe Prick test should only be used for latex and povidone. Both the Prick and Intradermal tests should be used for the remainder of drugs. It is better to carry out these tests no later than 6–12 months afterwards, although in the case of NMBAs, these can remain positive for several years.
Good sensitivity exists for: NMBAs, synthetic gelatines, β-lactams, chlorhexidine, latex and povidone.
Poor sensitivity occurs with barbiturates, opiates (which can give false positive because they release histamine) and benzodiazepines.
NMBAs: All of these should be tested given their high cross-reactivity, and in the future only those with a negative result will be used. The prick test is more useful for identifying what is responsible, and the intradermal test for evaluating cross-reactivity.
Controlled exposure testIf indicated, this will be used for: β-lactams, local anaesthetics, NSAIDs and latex.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this article.
Data confidentialityThe authors declare that no patient details appear in this article.
Privacy rights and informed consentThe authors declare that no patient details appear in this article.
Conflict of interestsThe authors declare that no economical or personal factors exist which could result in a conflict of interests with respect to the published article.