The incidence of perioperative anaphylactic reactions is overall estimated to be 1 per 10,000–20,000 anaesthetic procedures. We performed a retrospective analysis of patients referred to a University Allergy Centre in Belgium with the suspicion of an allergic reaction during or shortly after general anaesthesia.
ObjectivesOur aim was to assess the causes of perioperative allergic reactions, to evaluate cross-reactivity among neuromuscular blocking agents (NMBA) and to analyze the diagnostic relevance of tryptase levels in the discrimination between IgE and non-IgE-mediated reactions.
MethodsA total of 119 patients, referred from 2007 to 2011 were included. The diagnostic protocol consisted in case history, serum tryptase measurements, immunoassays and skin tests.
ResultsA diagnosis of IgE-mediated reaction was established in 76 cases (63.9%). The most common agents were NMBA (61.8%), antibiotics (14.5%), latex (9.2%) and chlorhexidine (5.2%). Rocuronium was the most frequently causative NMBA (48.9%). Vecuronium cross-reactivity was established by skin testing in 47.6% of cases. Cisatracurium was the NMBA most frequently tolerated (cross-reaction in 13.9%). In 23.4% of NMBA allergic patients, the reaction occurred on the first exposure. Most IgE-mediated reactions occurred during the induction phase (72.4%). Latex-induced reactions occurred mainly during maintenance and recovery phases (71.4%; p<0.02). Mean tryptase values were significantly higher in patients with IgE-mediated reactions (p=0.0001), than in those with no identified cause.
ConclusionsNMBA, antibiotics, latex and chlorhexidine were the main culprits of IgE-mediated perioperative reactions. Uncertainties remain concerning the specificity and sensitivity of skin testing. Tryptase assays can be useful in the discrimination of IgE and non-IgE-mediated reactions.
Complications during general anaesthesia have multiple causes, among which allergic reactions are rare. However, as they are a potential cause of perioperative morbidity and mortality, all possible allergic reactions should be carefully investigated.1–5 Data on the incidence of perioperative anaphylaxis have already been published in France,6–10 Australia,11 Scandinavia12–14 and England.15,16 The anaphylaxis rate varies between reports but it is overall estimated to be 1 per 10,000–20,000 anaesthetic procedures.7,10,17 Thirty to 60% of cases are due to IgE-mediated reactions with a 3.5–10% mortality rate.18 The most commonly involved agents are NMBA (50–90%), latex (4–30%) and antibiotics (5–20%).10,11,13 In other reports latex and antibiotics were identified as the main culprits.17,19 Dyes, hypnotic agents, local anaesthetics, opioids, colloids, aprotinin, protamine, chlorhexidine, and contrast agents are less frequently involved. With some of these agents (e.g. non-steroidal anti-inflammatory agents, opioids and contrast agents), non-immune mechanisms are more likely to be involved than IgE-mediated reactions.18–20
Diagnosis of perioperative allergic reactions is not always straightforward and the level of certainty in this area is still limited since provocation tests, which are the gold standard in drug allergy diagnosis, are contraindicated or impossible with many anaesthetics.4,5,21,22 Skin tests remain the gold standard for the detection of type I reactions. Some authors question the accuracy of these tests and point out the occurrence of false-positive results with intradermal testing due to non-specific histamine release by some agents with inappropriately higher concentrations (e.g. NMBA and opioids) or due to cross-reactions with other agents, such as cough medication.23–26 For this reason, although recommendations regarding skin tests procedures have been published, validation is still lacking.14,21–23 Laboratory tests include baseline and acute episode serum tryptase levels, specific IgE assays and cellular assays (e.g. histamine release assay, basophil activation test and cellular antigen stimulation test).4,5,21,22 Although such in vitro tools have been proposed in clinical practice, they are not available in many centres. Specific IgE assays have been developed for some drugs (e.g. suxamethonium, some beta-lactam antibiotics, thiopental, propofol, chlorhexidine and aprotinin), but they seem to be less sensitive than skin tests. Basophil activation test has been proposed for the indirect detection of specific-IgE antibodies, particularly when skin tests are difficult to interpret, but they also require further investigation.4,5,21,22
We carried out a 5-year retrospective study of the clinical data of all patients with probable perioperative hypersensitivity reaction referred to our University Allergy Centre from 2007 to 2011. Our aim was to compare the causes of perioperative allergic reactions to that in other countries, to evaluate cross-reactivity among NMBA and to analyze the diagnostic relevance of tryptase levels in the discrimination between IgE and non-IgE-mediated hypersensitivity reactions.
Materials and methodsAll patients with perioperative reaction possibly due to IgE-mediated allergy during or immediately after general anaesthesia were included (i.e. dermal signs, such as generalized erythema, urticaria or angioedema; respiratory disturbances, such as cough, bronchospasm, increased airway resistance or difficulty with ventilation; cardiovascular signs, such as hypotension, tachycardia, bradycardia, arrhythmia and cardiac or respiratory arrest).4,5 The diagnostic workup included medical history (age, sex, number of previous interventions, history of other allergic diseases), serum tryptase (Uni-CAP Tryptase, Phadia, Sweden) during the adverse reaction (<2h) and at a later time point (several days/weeks after), and specific-IgE assays for latex, penicillin, amoxicillin and ethylene oxide (Immuno-CAP System, Phadia, Sweden). Data on the clinical characteristics and timing of the reaction as well as drugs administered during surgery were obtained from anaesthetic record charts. An elevated tryptase level was defined as >11.4μg/L, according to the reference value provided by the company.
All patients underwent skin tests with the drugs and/or substances administered during the perioperative period and suspected of being involved in the reaction, including latex (commercial extracts for skin prick testing from ALK and Stallergenes). Skin tests were performed according to standardized diagnostic procedures, at least 4–6 weeks after the initial episode.4,5
In case of a positive reaction to the NMBA used for the anaesthesia, skin tests were also performed with other available NMBA, in an attempt to find an alternative drug for future procedures.
The diagnosis of an IgE-mediated reaction was suspected on the basis of the skin test and/or IgE assay results consistent with the type of reaction and the anaesthetic protocol. Statistical analysis was performed with GraphPad Prism 5 for Windows (GraphPad Software, Inc.). Fisher's exact test was used as the measure of contingency. D’Agostino and Pearson omnibus normality test was used for serum tryptase data. After logarithmic scale conversion, paired and unpaired t-test were used ad hoc.
ResultsA total of 119 patients were included, with female preponderance (61.3%) and with ages ranging from 15 to 81 years (mean age of 49.8 years). All patients had undergone general anaesthesia with NMBA.
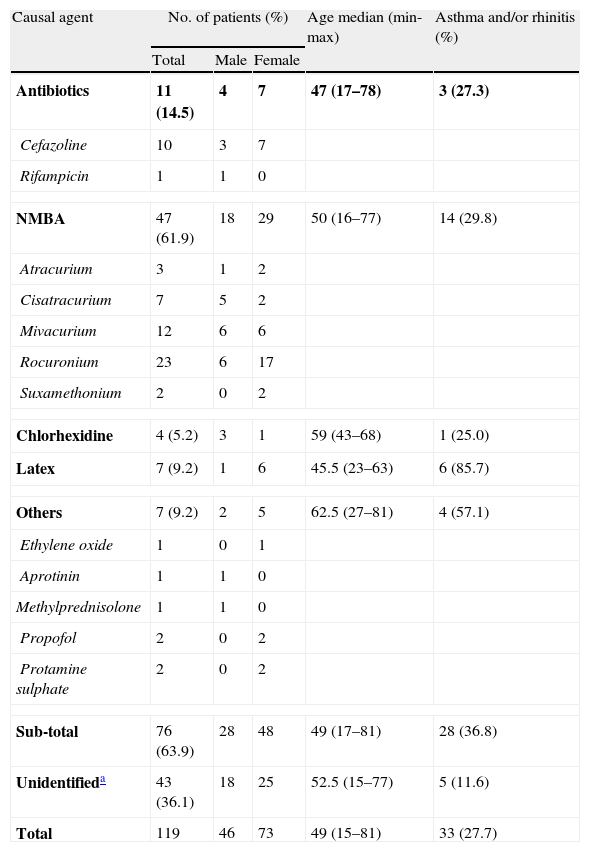
Identification of IgE-mediated allergic reactionsA diagnosis of IgE-mediated reaction was established by skin tests and/or ImmunoCap in 76 cases (63.9%). The remaining 43 cases (36.1%) were considered as non-IgE-mediated reactions (possibly non-immune as well) and classified accordingly as “unidentified cases” (see Table 1).
Causal agents in perioperative reactions (n=119).
| Causal agent | No. of patients (%) | Age median (min-max) | Asthma and/or rhinitis (%) | ||
| Total | Male | Female | |||
| Antibiotics | 11 (14.5) | 4 | 7 | 47 (17–78) | 3 (27.3) |
| Cefazoline | 10 | 3 | 7 | ||
| Rifampicin | 1 | 1 | 0 | ||
| NMBA | 47 (61.9) | 18 | 29 | 50 (16–77) | 14 (29.8) |
| Atracurium | 3 | 1 | 2 | ||
| Cisatracurium | 7 | 5 | 2 | ||
| Mivacurium | 12 | 6 | 6 | ||
| Rocuronium | 23 | 6 | 17 | ||
| Suxamethonium | 2 | 0 | 2 | ||
| Chlorhexidine | 4 (5.2) | 3 | 1 | 59 (43–68) | 1 (25.0) |
| Latex | 7 (9.2) | 1 | 6 | 45.5 (23–63) | 6 (85.7) |
| Others | 7 (9.2) | 2 | 5 | 62.5 (27–81) | 4 (57.1) |
| Ethylene oxide | 1 | 0 | 1 | ||
| Aprotinin | 1 | 1 | 0 | ||
| Methylprednisolone | 1 | 1 | 0 | ||
| Propofol | 2 | 0 | 2 | ||
| Protamine sulphate | 2 | 0 | 2 | ||
| Sub-total | 76 (63.9) | 28 | 48 | 49 (17–81) | 28 (36.8) |
| Unidentifieda | 43 (36.1) | 18 | 25 | 52.5 (15–77) | 5 (11.6) |
| Total | 119 | 46 | 73 | 49 (15–81) | 33 (27.7) |
Gender differences were not statistically associated with either the reaction mechanism or the causative agent. A statistically significant association was found between atopy and IgE-mediated reactions (p<0.005). As for the clinical manifestations in patients with allergic reactions, dermal symptoms (erythema, urticaria or angioedema) were reported in 81.6%, followed by cardiovascular symptoms in 72.4% (hypotension, tachycardia or bradycardia, arrhythmia or cardiac arrest) and respiratory symptoms in 34.2% (cough, bronchospasm, increased airway resistance or difficulty inflating). Isolated dermal symptoms occurred in 15.8% of cases.
The most frequent cause of perioperative allergic reactions was NMBA (61.9%), followed by antibiotics (14.5%), latex (9.2%) and chlorhexidine (5.2%). Among patients with NMBA allergy (n=47), the most common culprit agent was rocuronium (48.9%).
All patient with latex allergy (n=7) had positive skin prick tests and positive specific-IgE assays (Immuno-CAP), 7.98±1.73kU/L. Latex allergy was statistically associated with a history of asthma and/or allergic rhinitis (p<0.005 and relative risk, RR of 3.17, 95% CI). After careful retrospective assessment of their medical history, 3 patients (42.9%) reported previous symptoms of fruit allergy or intolerance to materials containing latex before surgery.
Antibiotic allergy was diagnosed in 11 patients, in all but one (i.e. rifampicin) due to cephalosporins (i.e. cefazoline). Diagnosis was established by skin prick testing and intradermal testing. None of these patients reported history of drug allergy.
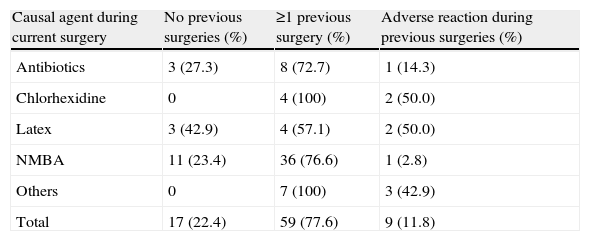
Most patients (77.6%) with perioperative allergic reactions had undergone 1 or 2 surgical interventions under general anaesthesia in the past. Nine patients reported adverse reactions during prior surgeries (9/59). Urticaria was the most common previous perioperative reaction reported by patients. Allergy to NMBA was diagnosed in 11 patients with no previous surgeries and no known previous exposures to NMBA (23.4%) (see Table 2).
Previous surgeries in patients with IgE-mediated perioperative reactions (n=76).
| Causal agent during current surgery | No previous surgeries (%) | ≥1 previous surgery (%) | Adverse reaction during previous surgeries (%) |
| Antibiotics | 3 (27.3) | 8 (72.7) | 1 (14.3) |
| Chlorhexidine | 0 | 4 (100) | 2 (50.0) |
| Latex | 3 (42.9) | 4 (57.1) | 2 (50.0) |
| NMBA | 11 (23.4) | 36 (76.6) | 1 (2.8) |
| Others | 0 | 7 (100) | 3 (42.9) |
| Total | 17 (22.4) | 59 (77.6) | 9 (11.8) |
Allergic reactions most frequently occurred during the induction/preparation phase (72.4%). Fifteen patients reacted during the maintenance phase and 6 during the recovery phase. Among NMBA allergic patients, most reactions occurred during the induction/preparation phase (p<0.0001 and RR of 2.21) while IgE-mediated reactions to latex occurred mainly in the maintenance and recovery phases (p=0.0152 and RR of 6.55).
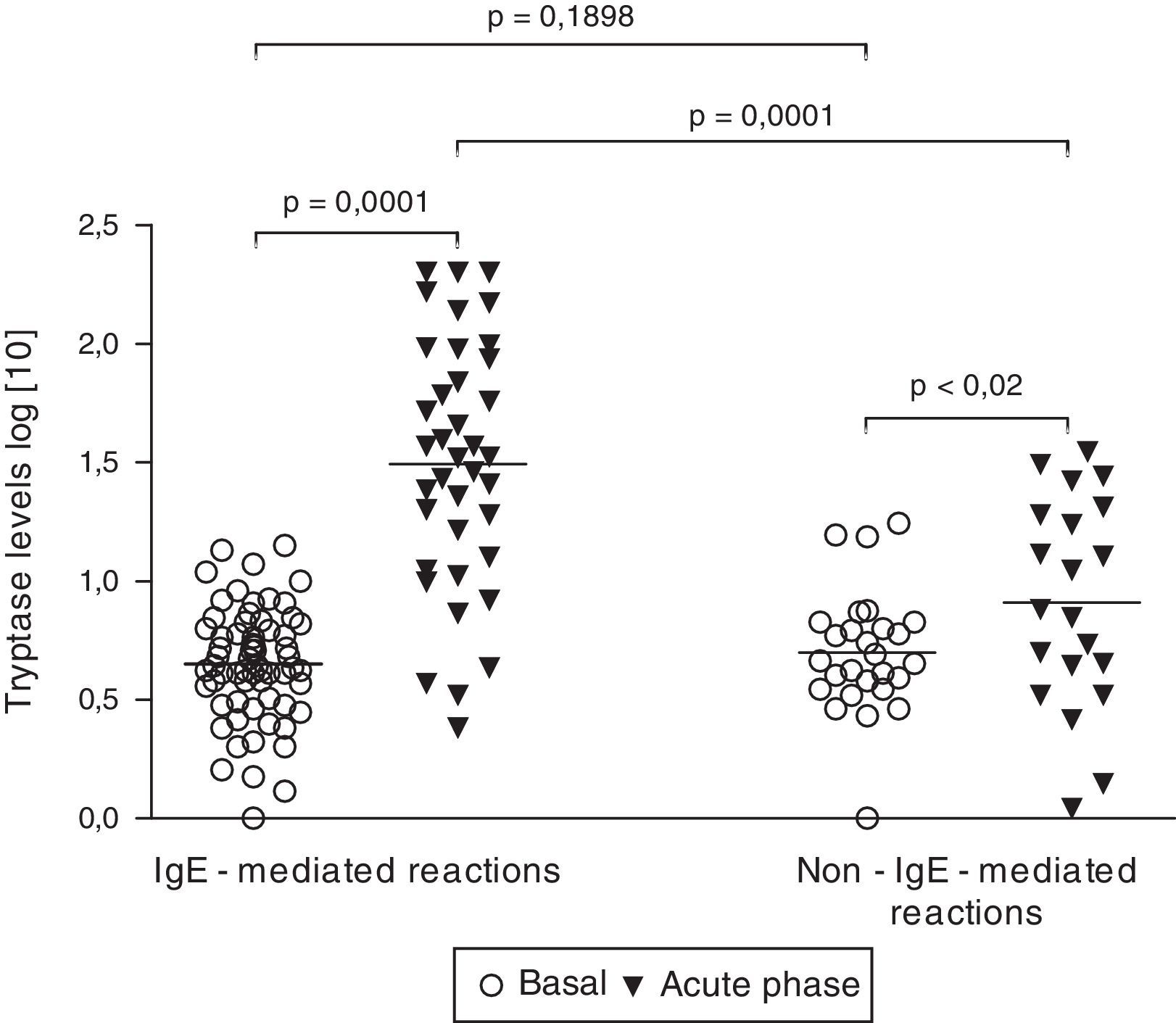
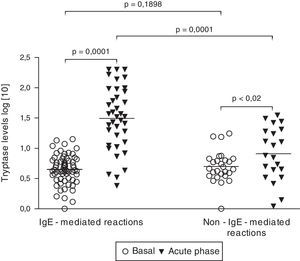
Tryptase levelsTryptase levels during the acute phase were obtained in 59 patients. Among them, 36 patients had experienced an IgE-mediated reaction (61.0%) and 23 patients had a non-IgE-mediated reaction (39.0%). Mean acute phase tryptase values were significantly higher in patients with IgE-mediated reactions (59.3±10.0μg/L versus 11.9±2.1μg/L) (p=0.0004). No significant difference was found between basal tryptase levels of patients with IgE and non-IgE-mediated reactions. Among patients with non-IgE-mediated reactions, tryptase levels during the acute phase were above the upper limit (>11.4μg/L) in 7 cases (30.4%). On the other hand, among patients with IgE-mediated reactions, tryptase levels were found to be increased in 29 cases, but to be within the normal range in 7 (19.4%). All patients with tryptase levels above 25μg/L had IgE-mediated perioperative reactions (Fig. 1).
Basal (n=90) and acute phase tryptase levels (n=59). Tryptase levels were measured with an immunoassay. Results did not follow a Gaussian distribution (D’Agostino and Pearson omnibus normality test). Significance was calculated after logarithmic scale conversion, by using paired t-test (basal versus acute phase levels in both IgE and non-IgE-reactions) and unpaired t-test (basal and acute phase levels in IgE-mediated reactions versus non-IgE-reactions).
Skin testing with inappropriately high concentrations can induce irritative reactions (i.e. false-positive results).
Although none of our patients (n=119) had been exposed to vecuronium, we found a high rate of positive reactions on intradermal testing with 1:10 dilution. Among patients without NMBA allergy and without previous exposition to vecuronium (n=26), intradermal test with 1:10 dilution induced a positive reaction in 22 cases (84.6%). With 1:100 or higher dilution, only 3 out of these 26 patients were positive. Mivacurium was tested in 14 patients not previously exposed to this drug and non-allergic to any other NMBA. Among them, 3 had a positive reaction with 1:1000 or 1:10,000 dilutions. With 1:100 dilution, 11/14 (85.7%) had a positive reaction. This high rate of false positive reactions with 1:100 dilution is in accordance with other literature data and we did not consider this concentration in our analysis.
In patients with NMBA allergy it is important to propose an alternative agent for eventual surgeries in the future. Therefore, besides the culprit NMBA we also tested these patients with an alternative NMBA.
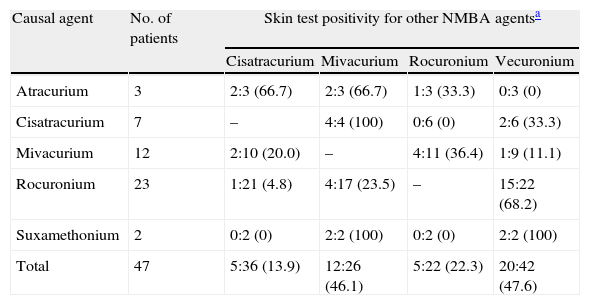
For the skin test assessment of cross-reactivity between NMBA, only the patients with proven NMBA allergy were considered (n=47) (see Table 3) and the highest concentrations for intradermal testing were 1:100 for cisatracurium, vecuronium and rocuronium, 1:1000 for mivacurium and suxamethonium. Vecuronium was tested as an alternative agent in 42 patients. A positive reaction was found in 20 patients and most of them (75.0%) had experienced a perioperative reaction with rocuronium. Mivacurium was tested as an alternative drug in 26 patients and 12 were positive. On the contrary, rocuronium and cisatracurium showed low cross-reactivity risk on skin testing (22.3% and 13.9% respectively).
NMBA cross-reactivity assessed by skin testing among NMBA allergic patients.
| Causal agent | No. of patients | Skin test positivity for other NMBA agentsa | |||
| Cisatracurium | Mivacurium | Rocuronium | Vecuronium | ||
| Atracurium | 3 | 2:3 (66.7) | 2:3 (66.7) | 1:3 (33.3) | 0:3 (0) |
| Cisatracurium | 7 | – | 4:4 (100) | 0:6 (0) | 2:6 (33.3) |
| Mivacurium | 12 | 2:10 (20.0) | – | 4:11 (36.4) | 1:9 (11.1) |
| Rocuronium | 23 | 1:21 (4.8) | 4:17 (23.5) | – | 15:22 (68.2) |
| Suxamethonium | 2 | 0:2 (0) | 2:2 (100) | 0:2 (0) | 2:2 (100) |
| Total | 47 | 5:36 (13.9) | 12:26 (46.1) | 5:22 (22.3) | 20:42 (47.6) |
Positive reactions: tested patients (%), using to the following drug concentrations: Cisatracurium dilution 1:100 or higher (prick test undiluted); Mivacurium dilution 1:1000 or higher (prick test 1:10); Rocuronium 1:100 or higher (prick test undiluted); Vecuronium dilution 1:100 or higher (prick test undiluted).
Similarly to what has been previously reported in the literature,6–16 NMBA were the leading cause of perioperative allergic reactions (n=47, 61.9%) in our series. Antibiotics represent the third cause in most reports. Our data shift antibiotic allergy to the second place (n=11, 14.5%). Cephalosporins (cefazoline in particular) were involved in all but one of these cases. This high prevalence could possibly be due to their widespread use in the context of general anaesthesia in Belgium, although we have no exact data on this issue. The impact of each causative agent on perioperative allergy will eventually change with time and can be different in other geographical areas, as it is influenced by the variable pattern of usage.10–13,27 In the past, suxamethonium was the most frequently reported cause of perioperative allergic reactions but it is now a rare cause of allergy as it is seldom used. Recent data instead suggest an increasing incidence of allergy to atracurium, rocuronium and cisatracurium.10,28 Our results also indicate a high incidence of allergy to rocuronium (48.9% of all NMBA allergic patients). This apparent increase might rather be due to an increased rocuronium market share, biased reporting of adverse effects of new drugs or possibly by false positive results on skin tests.10,27–29 It should be mentioned that we have no data on the market share of NMBA in Belgium. Mertes et al. classified suxamethonium and rocuronium as drugs with a high risk of sensitization, pancuronium and vecuronium as having medium risk and atracurium and cisatracurium as having a low risk.8 These conclusions could be reached by relating the occurrence of allergic reactions to the frequency of use for anaesthesia.
Cross-reaction occurs commonly among NMBA (60–70% of the patients) and demands special attention regarding the selection of an alternative drug.8,28,29 It is recommended to perform skin tests with several commercially available NMBA in order to increase the probability of finding a safe alternative.4,5 According to Mertes et al. vecuronium has the highest risk of cross-reaction (87.5%) followed by rocuronium (80.6%), atracurium (76.8%) and suxamethonium (54.3%).8 In another study, rocuronium was the least cross-reactive, with negative skin-tests in one-third of the patients with NMBA allergy.28 We also found vecuronium (at the 1:100 or 1:1000 dilution) to have the highest cross-reactivity rate assessed by skin testing (47.6%). On the contrary, rocuronium and cisatracurium showed low cross-reactivity risk (22.3% and 13.9%, respectively).
Although skin tests were performed with ‘non-irritant’ concentrations according to international protocols, a significant number of patients reacted to vecuronium at the 1:10 dilution. These were probably irritative reactions (i.e. false-positive results) as none of these patients received vecuronium during present or past surgeries and none reacted to any NMBA other than vecuronium. This raises questions on validation of skin test procedures. We therefore conclude that 1:10 dilution of vecuronium induces a high rate of false-positive reactions and higher dilution factors (i.e. 1:100 or more) should be used. Low concentrations however may compromise the sensitivity of the diagnostic procedure.
Among patients who received mivacurium during surgery (n=26), 11 had positive reaction with the prick test or with intradermal test at 1:1000 or 1:10,000 dilutions and were accordingly considered allergic to mivacurium. The other 15 patients reacted with 1:100 dilution (intradermal test) and in 2 of these cases another cause for the perioperative reaction was found (antibiotics). In view of the false positive reactions with this dilution as already pointed out by Mertes et al., we could not ascertain the diagnosis of mivacurium allergy in these 13 subjects, and considered them as negative.23 Whether or not these patients were allergic to mivacurium remains uncertain. Therefore, more research is still required in order to avoid diagnostic errors.
Seven of our patients had latex allergy. Atopy, female gender, professional exposure, repeated surgeries or procedures involving natural rubber latex products, especially in the first year of life, constitute important risk factors for latex allergy.30 Our data indicate that risk factors for latex allergy could have been identified in 3/7 patients by a more careful history (latex-fruit syndrome in 1 patient and adverse symptoms with latex products in 2 patients), drawing attention to the need for a specific preoperative screening instead of a standard preanaesthetic questionnaire.4,5,10
Most patients with IgE-mediated reactions had previous exposure to anaesthetic drugs. Reactions occurred upon first contact in 17 patients (22.4%), a rate higher than seen in France (12.3%).10 Most cases (64.7%) were attributed to NMBA hypersensitivity. According to literature, allergic reactions to NMBA can occur on the first exposure in 15–50% of cases.6–13 Sensitization to quaternary amine epitopes, the major allergenic determinants in NMBA, might be the result of cross-reaction pathways.31 Sex hormones have been suggested to have immunomodulatory properties, skewing the immune response towards a Th2 profile and may, therefore, play a role in drug sensitization. This could explain the higher prevalence of IgE-mediated perioperative anaphylaxis in female patients.32
The timing of reaction is, to some extent dependent on the culprit agent and the route of exposure. Antibiotics are often given intravenously at induction, commonly within a minute of induction agents and a rapid onset of symptoms is usually reported. The same applies to NMBA. With latex, the allergens are absorbed through the peritoneum, respiratory mucosa or skin and symptoms tend to develop more slowly.20 In line with previous reports, we found significant differences in time of onset of symptoms, with NMBA allergy causing symptoms during the induction/preparation phase and latex allergy being responsible for later phase reactions.
Concerning clinical features, dermal symptoms are the most commonly reported but may be absent in about one-third of patients, particularly in cases of rapidly progressive anaphylaxis.10,19 We found 22.4% (n=17) patients with IgE-mediated reaction with no skin involvement. Therefore, the absence of dermal symptoms should not preclude an allergic mechanism.4
Serum tryptase elevation can occur in both IgE-mediated and non-IgE-mediated reactions and does not help to identify the specific cause. In a study of 712 patients with perioperative allergic reactions, the positive predictive value of tryptase for the diagnosis of perioperative anaphylaxis (IgE-mediated mechanism) was 95.3% and the negative predictive value was 49%.9 According to our results, mean tryptase values during acute reactions were significantly higher in patients with IgE-mediated reactions. At a cut-off value of 25μg/L instead of 11.4μg/L, serum tryptase is highly suggestive of an IgE-mediated reaction.4.
Regarding the limitations of our work, the data on atopy were based exclusively on a history of eczema, asthma and/or rhinitis. This constitutes a relevant limitation for the analysis of atopy as a risk factor for perioperative allergy. The data on timing of reaction and clinical manifestations were not available in all cases due to inappropriate reports in anaesthetic charts. Tryptase data (i.e. acute phase or basal assessment) were not available for some patients, and blood samples during the acute reaction were taken in the first 6h but not necessarily all at the same time point in relation to the acute reaction.
Globally, these results support an active policy of risk reduction, with control of unnecessary exposure to potentially sensitizing compounds (i.e. quaternary amine epitopes) and a systematic search for eventual risk factors before anaesthesia (e.g. latex allergy and latex-fruit syndrome). Further, it is crucial to raise awareness among anaesthesiologists and surgeons for identifying patients with history of allergic perioperative reactions and to perform a proper evaluation before eventual reexposure. Intradermal tests should be performed and interpreted only by experienced physicians and the assessment of non-irritative concentrations, in particular for NMBA, should receive more attention in the future research.
Brief summary statementIn a retrospective analysis of 119 patients with perioperative hypersensitivity reactions, an IgE-mediated allergy was diagnosed in 64% of cases. Neuromuscular blocking agents remain the agents most likely to cause an IgE-mediated reaction. Antibiotics were more frequently involved than latex and some rare causes were also identified. The authors raise a number of concerns about validity and sensitivity of drug skin testing.
Ethical disclosuresPatients’ data protectionThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Financial supportGrant from the Study Center for Allergy Projects (SCAP), Harlem, The Netherlands.
Conflict of interestThe authors have no conflict of interest to declare.
This work was supported by a grant from the Study Centre for Allergy Projects (SCAP), Harlem, The Netherlands.