Visual Analogue Scale (VAS) has been proposed as a useful tool for assessing the perception of asthma symptoms, a cornerstone in disease management. While airway flow limitation and its reversibility are thought to be a useful marker of disease severity, there are very few studies that evaluated the response to bronchodilation (BD) testing perception by VAS. To investigate whether VAS assessment of breathlessness perception could provide a useful tool to assess the response to BD testing in asthmatic children.
MethodsThis cross-sectional study included a total of 150 children (96 males, mean age 11.05 years) with asthma, 50 had bronchial obstruction (i.e. FEV1 <80% of predicted). Perception of breathlessness was assessed by VAS; lung function was measured by spirometry. BD testing was performed in all children.
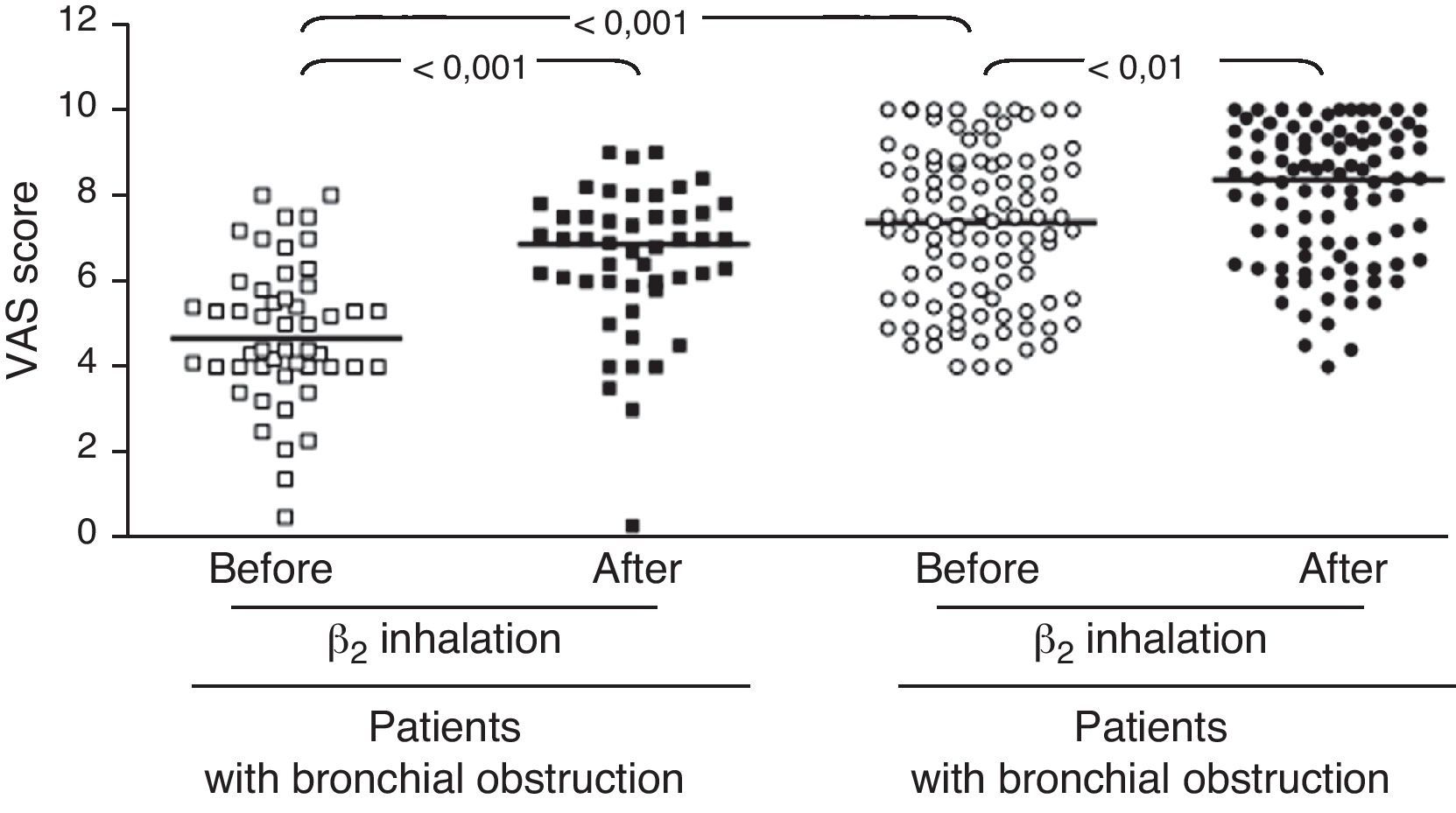
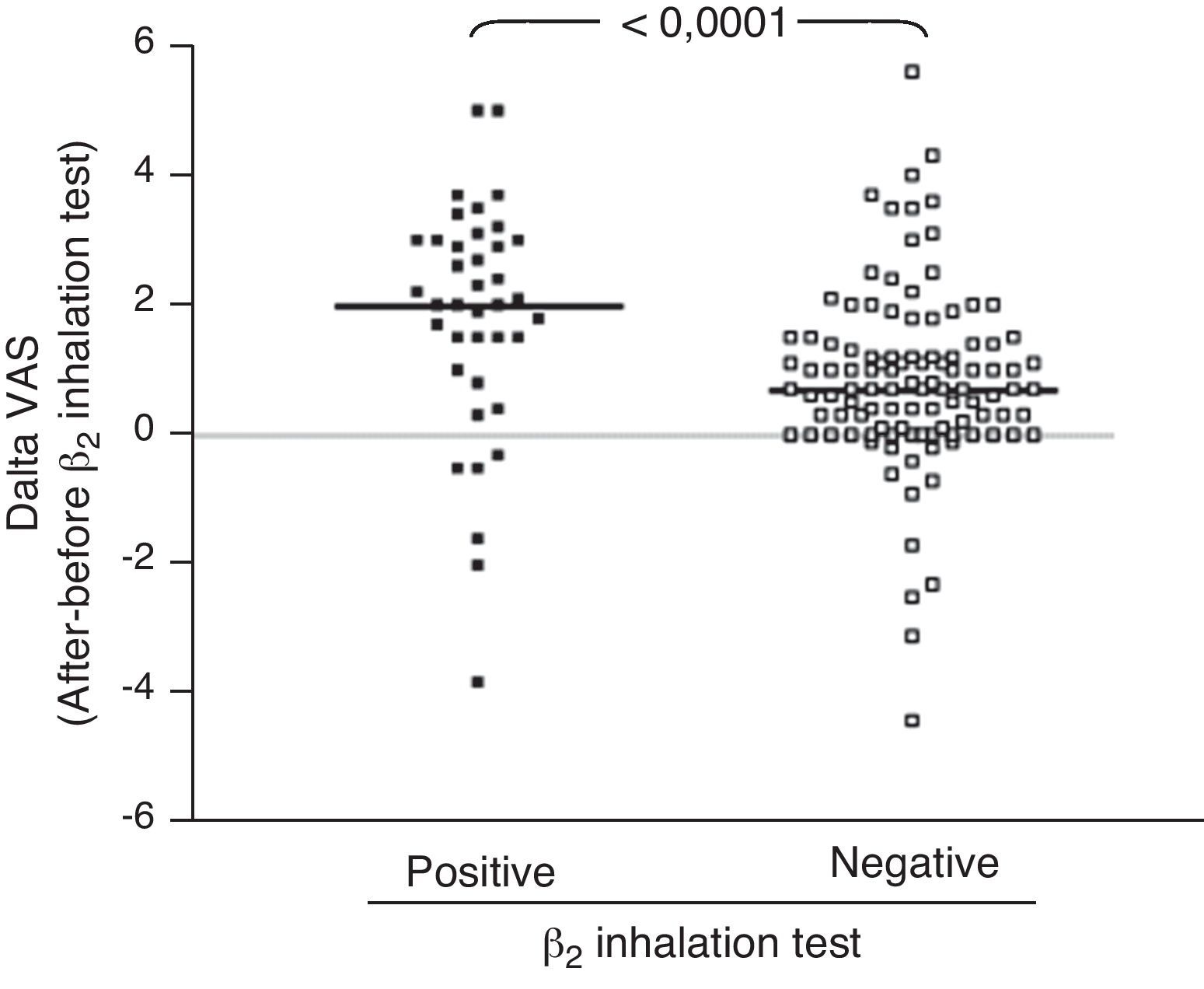
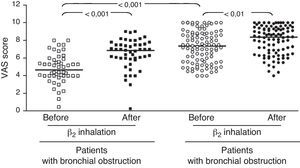
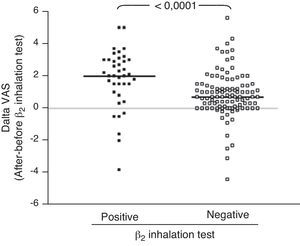
ResultsIn children with bronchial obstruction, VAS at baseline was 4.7 and significantly increased to 6.9 (p<0.001) after BD. In children without bronchial obstruction, VAS at baseline was 7.4, but further significantly increased to 8.4 after BD testing (p<0.01). There was a significant difference in Δ VAS between children with bronchial reversibility and children without it (p<0.0001).
ConclusionsThe present study demonstrates that VAS might be considered an initial tool to assess the BD response in children with asthma, mainly with overt bronchial obstruction.
Asthma is characterised by two main pathophysiological characteristics: chronic bronchial inflammation and bronchial hyper-responsiveness to a variety of stimuli, both of them inducing airway obstruction and consequently symptom occurrence, mainly breathlessness.1 Reversibility of airflow obstruction is the pathognomonic characteristic of asthma: in fact, bronchodilation testing is commonly used to confirm the asthma diagnosis. However, this test is usually performed only in specialised centres and so it is rarely accessible to the majority of asthmatic patients. The possibility of assessing bronchial reversibility using a simple tool such as VAS could be clinically relevant as it might allow healthcare providers to obtain this information in non-specialty healthcare settings. The control of asthma symptoms is actually considered the cornerstone goal in the management strategy and the level of the achieved control serves also to classify asthma severity. However, many children with asthma are not referred for lung function assessment and do not obtain a well-tailored treatment. Therefore, most of them are managed by family paediatricians who usually base the treatment decisions on symptom report and clinical examination.
Asthmatic patients vary in their ability to perceive the airway obstruction.2 In fact, fairly large discrepancies have been noted between patients’ subjective ratings of the severity of impaired pulmonary functioning and objective measures of lung function.3–5 In particular, the paediatric age is characterised by the additional problem of one individual (the child) experiencing the symptom, and another individual (the parent) needing to interpret the symptoms to decide (or at least participate in deciding) on the course of management. However, judgments of symptom severity are only one aspect of symptom perception and management.6 Laypeople cognitively organise information concerning physical symptoms according to prototypical conception about specific disease.7 Thus, symptoms not behaving to this prototypical representation may be ignored.3 It was reported that asthmatic patients may ignore early symptoms of exacerbation and easily confuse asthma symptoms with medication side effects.8 Beyond perceiving symptoms, patients and parents evaluate and interpret them in a larger context of illness meaning.3
Much of the research on symptom perception focused on relating the accuracy of the patients’ subjective symptom perception to the physiological response objectively measured by spirometry. However, in addition to the physical symptom parameters, several factors may exert a role in symptom perception, including: (i) past experience with asthma attacks and the criterion level for action that the patient and family has established; (ii) other background noise from which the symptom has to be discriminated, such as competing symptoms from medication side effects, anxiety, or inter-current illness; and (iii) other evaluative, cultural, and affective components.3 Furthermore, it has been proposed that symptom perception and evaluation by children (and parents) has to be considered a multidimensional construct consisting of: (i) accuracy of the assessment of the physical parameter of the symptom (e.g. how tight am I?); (ii) discrimination about what constitutes a symptom related to asthma; (iii) evaluation of the level of symptom intensity at which an intervention is necessary; and (iv) negotiation between child and parent.3
The breathlessness perception may be measured by the Visual Analogue Scale (VAS). The validity of VAS was previously evidenced in the measurement of the sensation of breathlessness in adults and children in both experimental and clinical studies.9,10 VAS has also been used to investigate breathlessness perception in children as young as five years11–13: it has been reported that VAS was sensitive in measuring differences between the means for good, usual, and bad breathing days. VAS was also considered useful in assessing symptom severity when compared with lung function testing.3 On the other hand, physical findings may be inadequate for assessing bronchial obstruction and remarkable airway obstruction may be present despite a normal clinical examination.14 Therefore, lung function assessment remains the best way to detect airflow obstruction.
The assessment of response to bronchodilation testing by VAS has been investigated by very few studies.15–17 It was demonstrated that VAS was a tool to obtain reliable information on breathlessness.18 It was hypothesised that the patient's breath perception, determined by their VAS score, could correlate with the degree of bronchial obstruction, as measured by the forced expiratory volume in 1sec (FEV1). Therefore, this study aimed at investigating whether VAS assessment of breathlessness perception could be useful in initially evaluating the response to bronchodilation testing in children with asthma, particularly in non-specialty settings.
Materials and methodsStudy populationThis cross-sectional study included a total of 150 children [96 males and 54 females, mean age 11.05 years] with asthma, who had been consecutively referred as outpatients to the Allergy Center of the G. Gaslini Institute for thorough asthma evaluation. The Institutional Ethical Committee of the G. Gaslini Institute approved the protocol. Signed informed parental consent and the child's assent (if the child was ≥12 years old) were obtained.
Data collectionInformation on demographics, asthma symptoms, and lung function, was collected at the time of the survey. Information on current asthma-related symptoms (breathlessness, chest tightness, wheezing, recurrent dry cough or exercise-related symptoms) was collected. The diagnosis of asthma was performed according to the Global Initiative for Asthma (GINA) guidelines (www.ginasthma.com).
Inclusion criteria were: (i) having asthma clinical diagnosis performed by the family paediatrician, and (ii) being aged between 6 and 18 years old (the minimum age of 6 years was chosen to ensure that children were able to perform reproducible lung function tests). Exclusion criteria were: (i) use of medium-high doses of inhaled corticosteroids (such as >200mcg of beclomethasone/daily or equivalent) or any systemic corticosteroids; (ii) current use of long acting β2 agonists; (iii) recent upper and/or lower respiratory infections; and (iv) insufficient knowledge of Italian language.
Sample size calculations were performed based on the primary outcome of the inter-group (patients with or without bronchial obstruction) difference in the change in VAS (post–pre). Power was set at 0.80 and alpha at 0.05. A sample size of 43 participants in the group of patients with bronchial obstruction and 86 in the group of patients without bronchial obstruction was required to detect a clinically meaningful change in VAS. Therefore, 50 children with overt bronchial obstruction (such as with FEV1 <80% of predicted) were compared with 100 well-matched asthmatic children without bronchial obstruction (such as with FEV1 ≥80% of predicted). Therefore, the study sample consisted of 150 subjects: 50 with bronchial obstruction and 100 without bronchial obstruction.
Visual Analogue Scale (VAS)The VAS consisted of one ruler asking for perception of breathlessness.15 Patients indicated their actual perception of breathlessness by marking a VAS. In this study, the VAS was a 10-cm vertical line on which 0 implied breathlessness, while 10 corresponded to no breathlessness. No interval marker was visible on the line. Patients were instructed to place a mark on the line indicating their ease of breathing at that moment. It was explained that 0 represented breathlessness and 10 no problem breathing. Thus, the lower the numerical score marked by the patient, the greater their perceived breathlessness. With a movable marker, the child could mark any point on the 10-cm segment which best described his/her perception.
VAS was recorded immediately before and after bronchodilation testingMeasurement of lung functionForced vital capacity (FVC), forced expiratory volume in 1s (FEV1) and forced expiratory flows at 25–75% of vital capacity (FEF25–75%), and the FEV1/FVC ratio were measured by spirometry (Med Graphics, Pulmonary Function System 1070 series 2, Med Graphics Corp., St. Paul, MN, USA), according to the guidelines provided by the American Thoracic Society and the European Respiratory Society.19 All the children were able to obtain at least three technically acceptable breathing manoeuvres with the spirometer. Three forced expiratory manoeuvres were obtained, and the best values were retained. The results were compared with reference values obtained from a well-defined population, identified by the American Thoracic Society and the European Respiratory Society, of healthy subjects comparable for gender, height, and weight and then expressed as a percentage.19
Bronchodilation testThe bronchodilation testing was performed according to international guidelines and using a salbutamol metered dose of 400mcg. Reversibility was considered if an increase of at least 12% of FEV1 from baseline was achieved, according to international guidelines.19
Statistical analysisThe distribution of each variable was checked using the Shapiro–Wilk W test. Descriptive statistics were performed and reported in terms of means with standard deviation (SD) (i.e. age) or medians with inter-quartile ranges (i.e. VAS, pulmonary function parameters). For comparisons between two groups, Mann–Whitney U test was used for non-normally distributed quantitative data. For comparisons among more than two groups, non-normally distributed quantitative data were analysed using Kruskall–Wallis test followed by Bonferroni's correction. The relationship between FEV1 (% pred.) and VAS was assessed by means of the Spearman's rank correlation coefficient. All tests were two-tailed and p values less than 0.05 have been considered as statistically significant. “Statistica release 8” (StatSoft Corp., Tulsa, OK, U.S.A.).
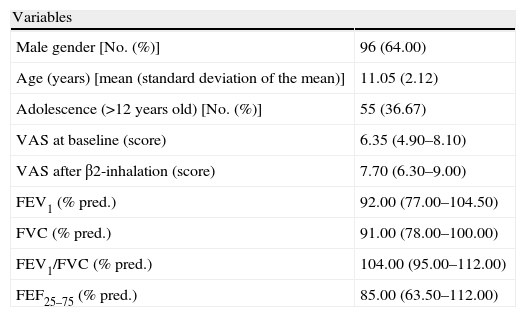
ResultsPatientsThe demographic, clinical, and functional characteristics of the patients recruited are reported in Table 1. There was a mild preponderance of male gender (64%), but without significance. Forty-one children showed reversibility after BD testing.
Demographic and clinical characteristics in the whole population.
| Variables | |
| Male gender [No. (%)] | 96 (64.00) |
| Age (years) [mean (standard deviation of the mean)] | 11.05 (2.12) |
| Adolescence (>12 years old) [No. (%)] | 55 (36.67) |
| VAS at baseline (score) | 6.35 (4.90–8.10) |
| VAS after β2-inhalation (score) | 7.70 (6.30–9.00) |
| FEV1 (% pred.) | 92.00 (77.00–104.50) |
| FVC (% pred.) | 91.00 (78.00–100.00) |
| FEV1/FVC (% pred.) | 104.00 (95.00–112.00) |
| FEF25–75 (% pred.) | 85.00 (63.50–112.00) |
All data are presented as median with lower and upper quartiles in parenthesis unless otherwise specified.
In the whole sample, the median VAS value was 6.35 at baseline and 7.7 after BD testing. Patients were firstly subdivided in two groups: those with and those without bronchial obstruction. Patients with bronchial obstruction had median VAS value of 4.7 (4–5.85) at baseline and 6.9 (5.95–7.55) after BD (p<0.001) as illustrated in Fig. 1. Children without bronchial obstruction had median VAS value of 7.4 (5.6–8.75) at baseline and 8.4 (6.6–9.4) after BD testing (p<0.01) as showed in Fig. 1. The intergroup analysis showed that the baseline VAS values were significantly different between the two sub-groups (p<0.01).
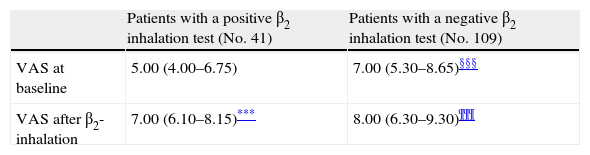
Further, children were subdivided into other sub-groups on the basis of the response to BD testing: those with or without bronchial obstruction reversibility (41 and 109 children, respectively) (Table 2).
VAS at baseline and after β2-inhalation in different subgroups of patients.
Patients with bronchial obstruction reversibility had median VAS value of 5 (4–6.75) at baseline and 7 (6.1–8.15) after BD (p<0.001). Children without bronchial obstruction reversibility had median VAS value of 7 (5.3–8.65) at baseline and 8 (6.3–9.3) after BD (p<0.001) as shown in Table 2. The intergroup analysis showed that the baseline VAS values were significantly different between the two sub-groups (p<0.01).
Analysing the Δ VAS (i.e. the difference in VAS score obtained after BD testing and before BD testing) there was a significant difference (p<0.0001) between children with bronchial reversibility and without it, as shown in Fig. 2.
Analysing the patients we found moderate positive correlations between VAS and FEV1 (r=0.491; p<0.0001). However, there was no significant relationship between VAS and age of children.
DiscussionThe perception of breathlessness has been investigated by several studies in children,21–27 but most of them were conducted in “experimental” settings, such as in patients with symptoms experimentally induced by bronchoconstrictor stimuli (such as methacholine, histamine, or exercise) also considering an ethnical aspect.20 Only one study was conducted in a real-life condition, such as considering the asthma symptoms perception assessed by VAS during a regular consultation in an outpatient clinic, where children with asthma were referred for the asthma diagnosis confirmation.18 On the other hand, very few studies investigated the response to BD testing using VAS score.15,17 The first study was mainly addressed to assessing the perception of airway obstruction induced by methacholine challenge and BD testing was performed after experimental obstruction.15 The second study was performed on adult patients with allergic rhinitis.17 Therefore, the present study was designed to confirm the possibility of using VAS for assessing perception of breathlessness in response to BD testing in children with asthma.
The present findings demonstrate that VAS assessment of breathlessness significantly decreased after BD in all patients. To better evaluate the usefulness of the VAS score, we enrolled two sub-groups of asthmatic children, such as patients with overt bronchial obstruction (FEV1 <80% of predicted) and children with normal lung function. The median VAS values were only significantly different at baseline: this finding underlines the ability of VAS assessment to discriminate the presence of bronchial obstruction. Children with or without bronchial obstruction perceived a statistically significant improvement of breathlessness VAS score after BD. Nevertheless, children with bronchial obstruction reported a higher VAS increase after BD: >2 units; whereas children without bronchial obstruction demonstrated a lower increase, i.e. about 1 unit. Therefore, VAS assessment of BD testing might discriminate subjects with overt bronchial obstruction if the VAS breathlessness measurement increases at least 2 units after BD.
Secondly, we investigated the VAS response to BD, considering bronchial reversibility. Also in this case, children with reversibility perceived a greater improvement of VAS breathlessness after BD. In fact, considering the Δ VAS values, children with reversibility reported a median increase of 2 units, whereas children without reversibility reported an increase <1 unit. Therefore, the simple assessment of the BD testing by VAS could allow to obtain raw information on bronchial reversibility, suggesting an asthma diagnosis both at home and at the paediatrician office. So it could suggest sending the child to specialised centres for deeper assessment. In addition, the moderate relationship between lung function and VAS could strengthen the applicability and utility of this instrument in settings where there is no spirometry equipment. In fact, many primary care practices do not have spirometry equipment or staff trained in the proper use and interpretation of results. As the VAS could be a reliable indicator of the child's perception of breathlessness, it could potentially be a valuable clinical assessment tool in these practices.
On the other hand, this study has a main limitation: as being conducted in a real-life setting, it did not include a large number of children with bronchial airflow obstruction. Therefore, further studies addressing this issue should be conducted.
In conclusion, the present study demonstrates that VAS might be considered an initial tool to assess the BD response in children with asthma, mainly with overt bronchial obstruction.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interests to declare.
Partially funded by Ricerca Corrente – Italian Ministry of Health.