Allergic rhinitis (AR) is a common disorder with social and economic impact. Daily activities, productivity, and quality of sleep may also be significantly affected by AR.1
The treatment of AR may be classified as: preventive, symptomatic, and allergen immunotherapy (AIT). However, environmental control measures are scarcely impressive and symptomatic therapy does not cure AR, as symptoms recur immediately after its suspension. In contrast, AIT treats all AR symptoms, including those of ocular allergy, by tackling the underlying cause of allergy, which consists of an aberration of the immune regulation. In fact, AIT is able of restoring the physiological immune tolerance towards the causal allergen.2 It has been evidenced that AIT is effective and exerts long-term preventive activity also after discontinuation.3 AIT is indicated in patients with AR when symptoms are surely IgE-dependent.
However, the assessment of AIT efficacy is not well standardised and is actually based on symptom severity and drug use. The measurement of AR nasal symptom intensity is usually performed by a 4-point scale (0=no symptom; 1=mild; 2=moderate; 3=severe). However, this methodology is based on a question made by the doctor restricted to some items. Recently, the Visual Analogue Scale (VAS) has been proposed to assess the severity of rhinitis symptoms.4 Therefore, the aim of this study was to evaluate the patients’ perception of AIT efficacy by assessing symptom severity and drug use with VAS in a cohort of patients suffering from AR.
The study was conducted in 20 Allergy and Rhinology Centres homogeneously distributed in Italy. The study was approved by the Review Board of each participating centre and an informed consent was obtained from each patient.
A total number of 198 patients (98 males, mean age 26.8 years) with AR were prospectively and consecutively enrolled. A detailed clinical history was taken and a complete physical examination was performed. The patients were included in the study on the basis of AR diagnosis performed according to validated criteria.1
Moreover, patients gave their perception of both the severity of symptoms and the use of drugs by VAS according to validated criteria.4 For symptoms, VAS must assess a global evaluation including all symptoms (for eye: itching, tearing and redness; for nose: itching, sneezing, rhinorrhea and obstruction). Antihistamines and intranasal corticosteroids were prescribed and the perception of their use was assessed by VAS. In this study, the VAS was a 10-cm horizontal line on which 0 implied no symptom or drug use, while 10 corresponded to very severe symptoms or maximal drug use. With a movable marker, the patient could mark any point on the 10-cm segment which best described his/her perception. No interval marker was visible on the line.
AIT was proposed as sublingual immunotherapy (SLIT) to all patients, although only 120 accepted it. The remaining 79 were treated with drugs alone and were considered as control.
Subjects undergoing sublingual immunotherapy (SLIT) received allergen commercial extracts (Stallergenes, Milan, Italy). SLIT schedule was a continuative course for two consecutive years. The most common allergen extracts used for AIT were: House Dust Mite (78), Parietaria (37), and grass (20).
The dose during the maintenance phase was five drops three times per week. Patients were visited at baseline (V1) and after two-year AIT (V2), assessing symptom severity and drug use by VAS.
Continuous and/or discrete parameters were reported as mean, SD, third quartile, and frequency. Categorical parameters were reported in contingency tables. Homogeneity of data was evaluated by χ2 Fisher exact test. The significance of the values concerning the principal parameters between V1 (at the moment of the first evaluation, before the start of AIT) and V2 (immediately after the AIT course) was calculated by Student's t test for paired data and by non-parametric Wilcoxon test for continuous parameters. Confidence Interval at 95% (CI) was reported for the mean of the differences between V1 and V2. McNemar test was used for categorical or discrete parameters. The p value concerning statistical significance was set at 0.05. Statistical analysis was performed by statistical package BMDP Dynamic produced by BMDP Statistical software Inc. (Los Angeles, CA, USA).
The AIT was well tolerated in all patients without clinically relevant adverse reactions. Drop-out rate was <5% in both groups.
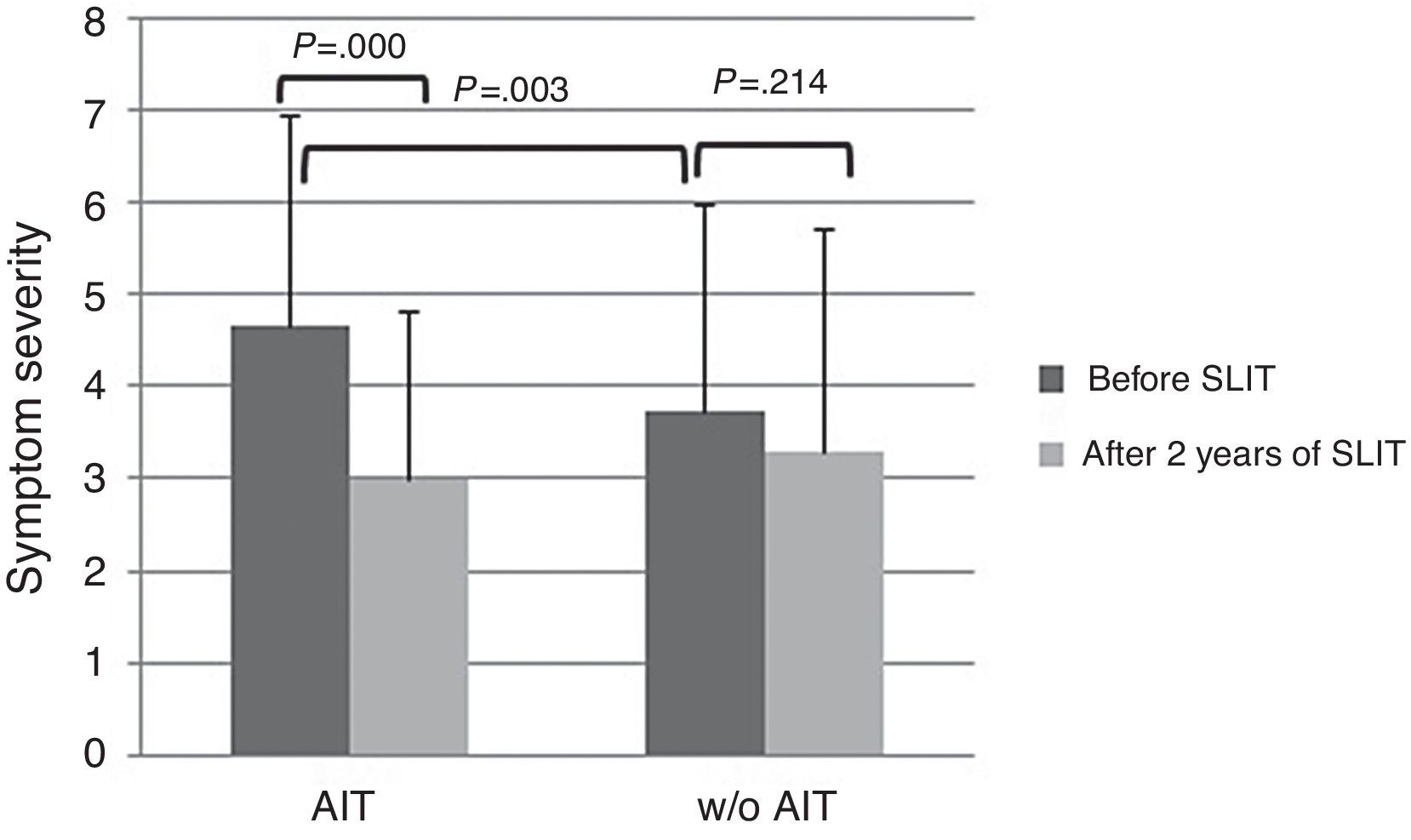
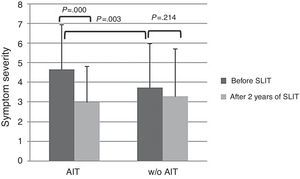
At baseline, AIT-treated patients perceived significantly (p<0.003) more severe symptoms (VAS 4.65±2.29) than control patients (VAS 3.73±1.84), as reported in Fig. 1. After AIT course, AIT-treated patients perceived a significant (p=0.000) reduction of symptom severity (VAS 3.0±2.25), whereas the control group did not perceive a change (VAS 3.31±2.42). There was a significant intergroup difference at baseline (p=0.003), whereas there was no difference after AIT course.
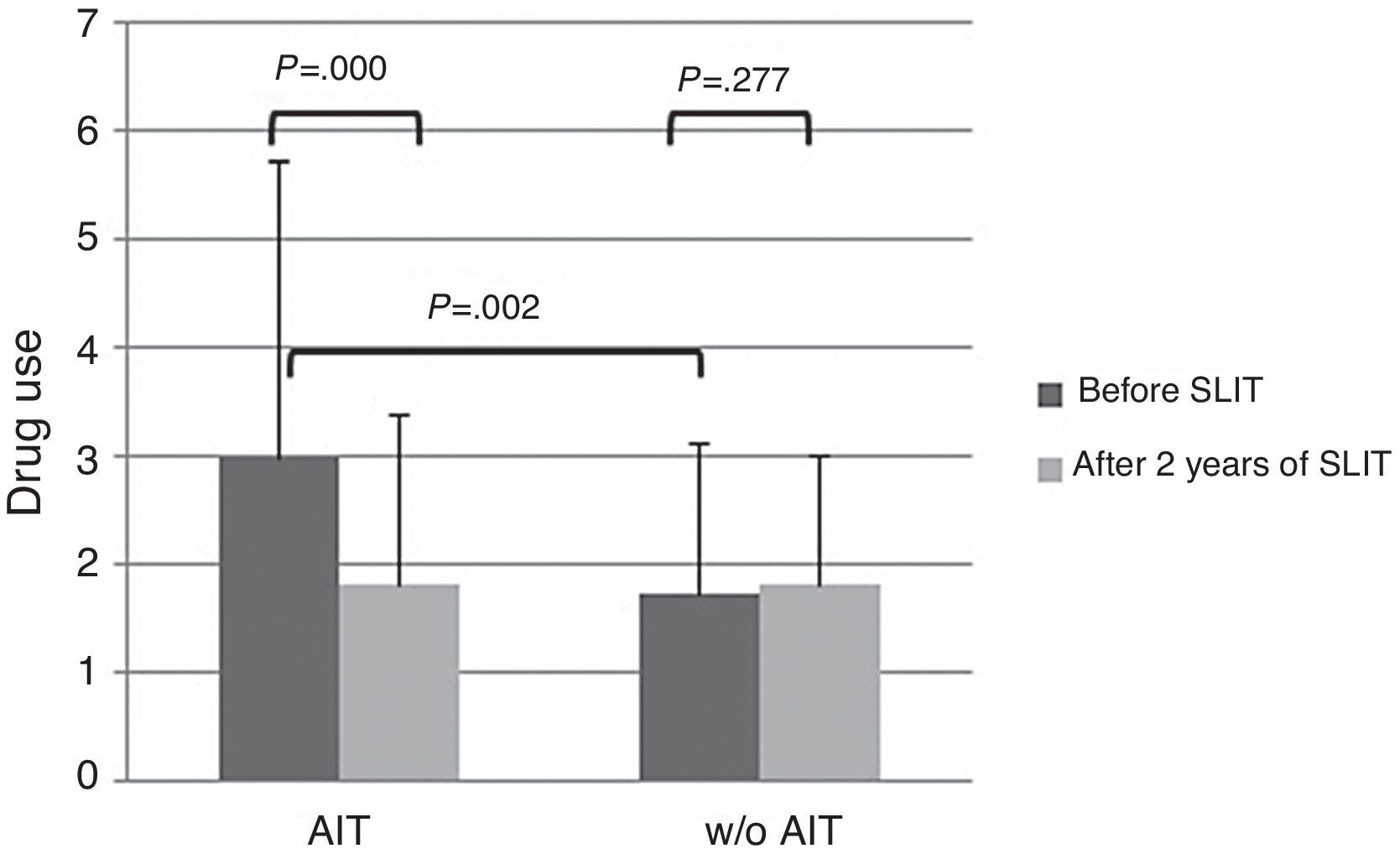
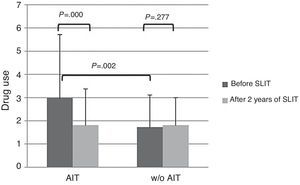
About drug use, AIT-treated patients perceived significantly (p<0.003) more drug use (VAS 3.0±2.72) than control patients (VAS 1.74±1.58) at baseline (Fig. 2). After AIT course, AIT-treated patients perceived a significant (p=0.000) reduction of drug use (VAS 1.81±1.39), whereas the control group did not perceive a change (VAS 1.82±1.2). There was a significant intergroup difference at baseline (p=0.002), whereas there was no difference after AIT course.
The present study, based on real-life clinical practice, provided some interesting outcomes concerning the patients’ point of view about the perception of symptom severity and drug use. Firstly, AIT-treated patients felt more intense symptoms and larger drug use than patients without AIT prescription. This finding underlines the concept that a criterion for identifying candidate to AIT might be the severity of AR. In this regard, it has been recently reported that the greatest improvement was observed in the patients with most severe AR symptoms.5
Secondly, at the end of two years immunotherapy, patients who had received AIT demonstrated a reduction in the perception of the severity of their symptoms, as well as a reduction in the perception of their drug-use, in comparison to the patients who only received symptomatic treatment. This point is interesting as it demonstrates that the perception of a patient may be a relevant approach to assess AIT efficacy. As there is no standardised outcome in evaluating AIT, to consider the patient perception may be useful in common practice.
The main limitation of the present study is that it was observational, as based on real life. Another critical point is that the two groups were mismatched at baseline as AIT-treated group reported more severe symptoms and more intense drug use. However, AIT-patients perceived a reduction of symptom severity of 35% and drug use of 40%, whereas control patients perceived only a slight improvement of symptoms (−10%) and the same drug use. Even though the significant difference between groups at the start may reduce the validity of the study and the possible placebo effect may account for about 25%, it is to note that only AIT-treated patients perceived an improvement both for symptom severity and drug consumption, whereas control patients did not report any relevant change. Moreover, the aim of this study was not the evaluation of AIT efficacy (well known and evidenced by rigorous trials), but the possibility of assessing the patients’ perception by VAS, a simple and validated tool.
In conclusion, this study shows that patients’ perception of symptom severity and drug use, assessed by VAS, may be a practical approach to assess AIT efficacy. In other words, the patient's point of view about the AIT efficacy evaluation may be a new way in the management of AR. However, further studies are needed to address this issue to fully confirm these findings.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
C Caffarelli (Università degli Studi di Parma, Dipartimento dell’Età Evolutiva, Parma), E Ridolo (Università degli Studi di Parma, Dipartimento di Scienze Cliniche), S Masieri (Department of Otorhinolaryngology, La Sapienza University, Rome), S Testi (Allergologia, Osp. S. Giovanni di Dio, Florence), C Valle (Allergologia, Azienda Ospedaliera San Paolo, Milan), G Pingitore (Allergologia, Ospedale GB Grassi, Rome), E Savi (U.O. Allergologia, Ospedale G. da Saliceto, Piacenza), C Romano (Servizio Prevenzione Diagnosi e Cura delle Malattie Allergiche e Respiratorie Azienda Sanitaria Locale – Napoli 5, Naples), N Mansi (AORN Santobono-Pausilipon, Naples), A Curcio (Allergologia, AO Santa Croce e Carle, Cuneo), L Armenio (UO di Pediatria S. Maggiore, AOU di Bari, Bari), A Fiocchi (Department of Pediatrics – Division of Allergy – Pediatric Hospital Bambino Gesù, Rome, Vatican City), L Terracciano (U.S.C. di Pediatria, Ospedale Fatebenefratelli, Milan), F Agostinis (U.S.C. di Pediatria, Ospedale Riuniti, Bergamo), G Capocasale (Pediatria, Ospedale San Giovanni di Dio, Crotone), E Gammeri (Pneumologia, AUSL 5, Messina), A Paternò and S Saporito (U.O.C. di Otorinolaringoiatria, AUSL 3, Catania), A Trigila (U.O. di Otorinolaringoiatria, AUSL 8, Siracusa).