This study was conducted to evaluate the oxidant/antioxidant balance (oxidative stress status) and plasma essential trace element levels in patients with bronchial asthma or allergic rhinitis.
MethodsA total of 94 individuals consisting of 19 allergic asthmatics; 17 non-allergic asthmatics; 22 patients with allergic rhinitis; and 36 healthy control people were enrolled into this study. Superoxide dismutase (CuZnSOD) and glutathione peroxidase (GSH-Px) activity as antioxidant defence mechanism parameters, along with malondialdehyde (MDA) as a marker of lipid peroxidation, were determined in erythrocytes of patient groups and controls. Plasma copper and zinc levels were also determined in all groups.
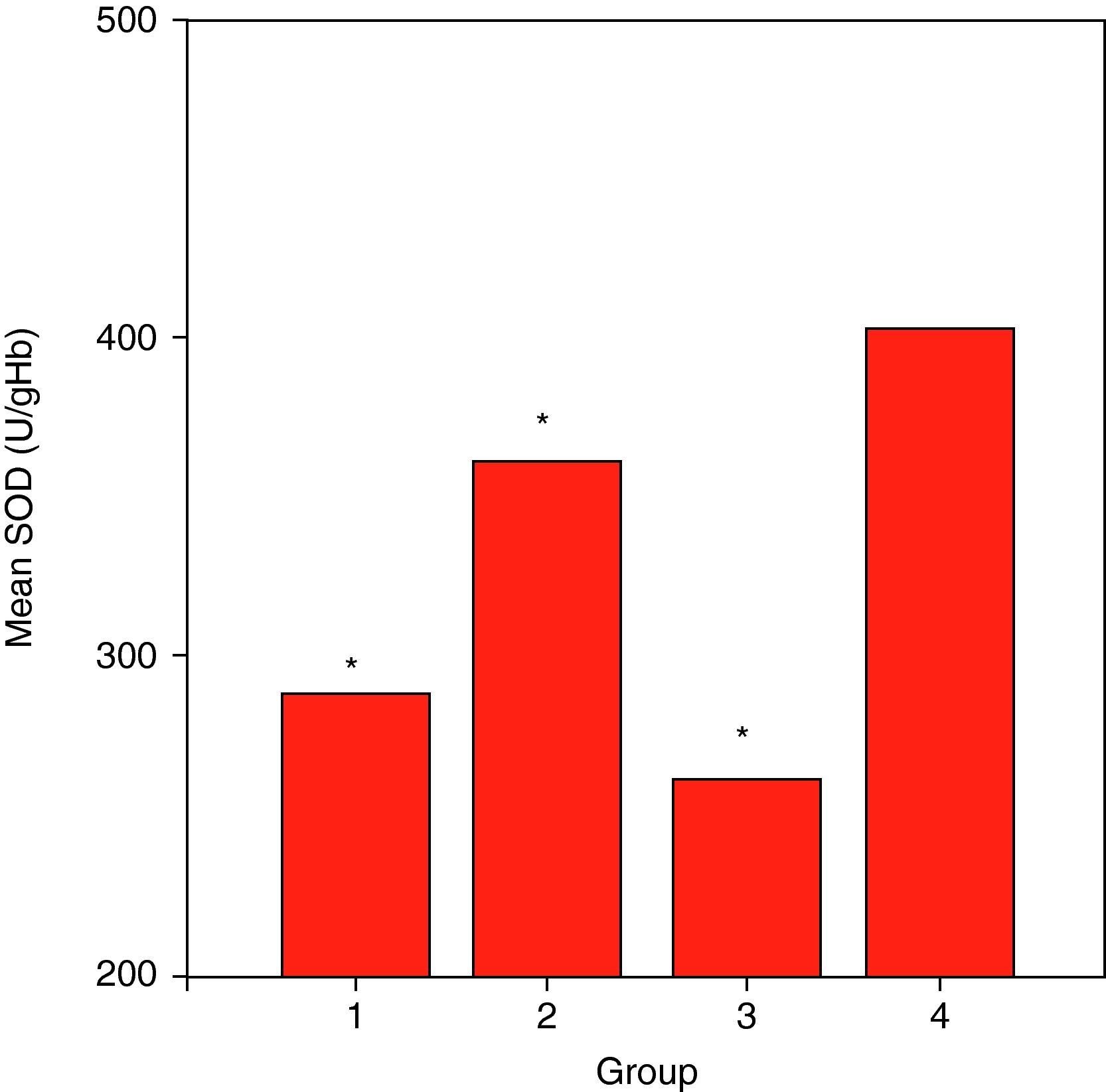
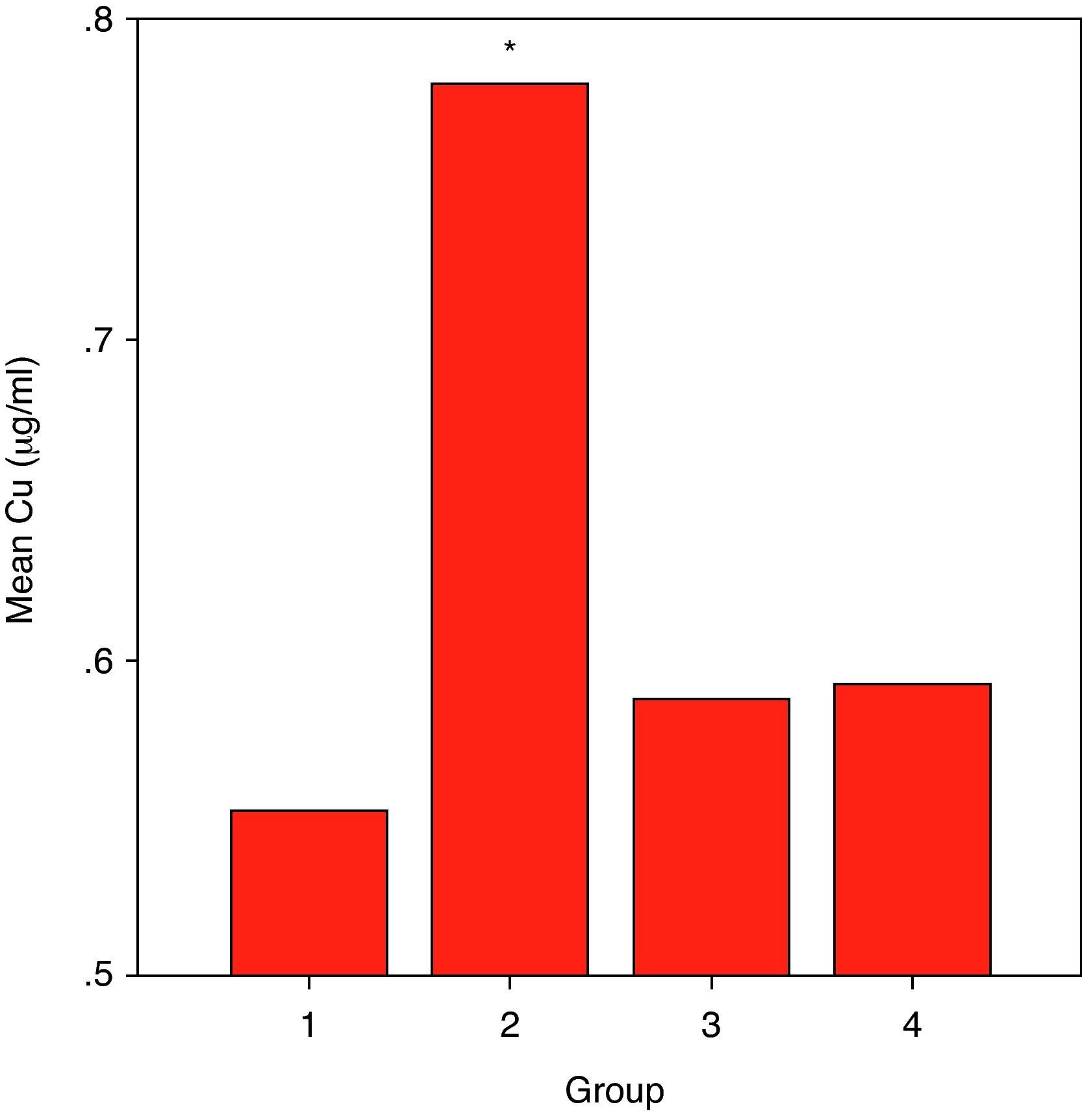
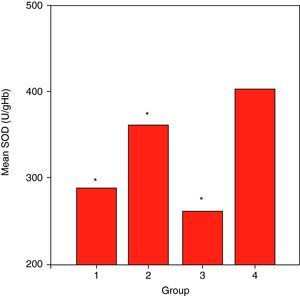
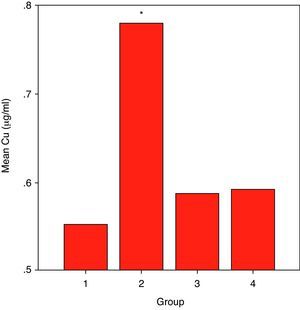
ResultsCuZnSOD activity was significantly lower in all groups of patients (p<0.001 for allergic asthmatics, p=0.008 for allergic rhinitis patients, and p<0.001 for non-allergic asthmatics) when compared to those of controls. Erythrocyte GSH-Px enzyme activity was not different when compared to that of the control group. Similarly, the patient groups had no difference from those of the controls with respect to erythrocyte MDA levels. While plasma Cu levels in all asthmatic patients were not different from those of the controls, allergic rhinitis patients had significantly elevated (p<0.001) Cu levels compared to those of the controls. No statistically significant difference was established between patient groups and controls with respect to plasma zinc levels.
ConclusionWhile defective CuZnSOD activity observed in all patients groups was expected to cause an increase in lipid peroxidation indicated by high MDA levels in these patients groups, the fact that MDA levels were not different from those of controls in all patient groups indicates that other components of anti-oxidant defence system preserve their functions in these patients. On the other hand, statistically significant difference between all patients groups and controls with respect to trace elements was only observed in allergic rhinitis patients who had higher levels of Cu than those of controls.
Asthma is the chronic inflammatory disease of airways in which mast cells, eosinophils and T lymphocytes take place.1 It can be allergic or non-allergic. Although scientific data and treatment facilities increase, this disease continues to cause significant morbidity and mortality. There are several risk factors for developing asthma in an individual. The dominant factor among these is the history of atopy. Bronchial hypersensitivity formed in atopic individuals is parallel to serum IgE.2 Bronchial hypersensitivity occurs in atopic individuals especially in case of sensitivity to more than one allergen. In previous studies, it has been shown that allergic rhinitis was present in 60% of the patients with asthma and asthma was present in 25-30% of the patients with allergic rhinitis preceding asthma symptoms (pre-asthma?).3 Therefore, togetherness of asthma and allergic rhinitis has revived the concept of “one airway one disease” with a continuity definition of a common airway inflammation.4
The role of free oxygen radicals (FOR), occurring in the inflammation area, on tissue damage and ethiopathogenesis of various diseases is gradually drawing more attention in medicine. Oxidative stress can be defined as exposure to increased oxidant or decreased antioxidant capacity.5
Inflammatory cells become active in patients with asthma and allergic rhinitis and produce too much FOR.6 This way, it was revealed that FOR, more accurately oxidative stress, can take part in the pathogenesis of allergic diseases like asthma and allergic rhinitis, chronic idiopathic rhinitis, and several studies have been and are still being conducted to enlighten this topic.7,8 While there are superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), GSH redox rings, there are also some non-enzymatic antioxidants like ceruloplasmin and vit-C, vit-E.9
Some trace elements take part as cofactors in the structure of these enzymes. Copper (Cu) and Zinc (Zn) are present in the structure of SOD. This enzyme converts O2 to H2O. Most of the CuZn SOD enzyme is found inside cell cytosol while being also found in lysosomes, between mitochondrial outer and inner membranes and in the nucleus. Also, there are many other enzymatic systems, in the structures of which, various trace elements like MnSOD, FeSOD, SeGSH-Px take part.10
The most important mechanism in the formation of tissue damage due to free radicals is the peroxidation of lipids.11 Oxidants launch lipid peroxidation by reacting with multiple unsaturated fatty acids (PUFA). Malondialdehyde (MDA), ethene, and pentane are revealed as final products,12 therefore, lipid structure is destroyed, permeability increases and cellular death occurs.13
In our study we aimed to investigate the role of oxidative stress particularly in the ethiopathogenesis of allergic diseases by determining plasma levels of Cu and Zn along with anti-oxidant activities in patients with allergic or non-allergic asthma and allergic rhinitis.
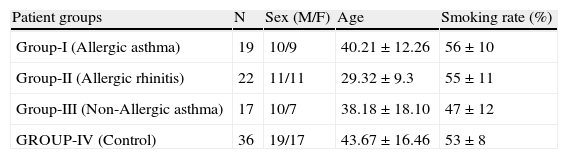
Materials and methodThis study was conducted between November 2004- May 2005 with the cooperation of Gulhane Military Academy of Medicine, Department of Internal Medicine and Central Directorate of Pharmaceutical Sciences. The study was approved by the local ethics committee of Gulhane School of Medicine. Asthma patients with positive prick test were grouped as allergic asthma group (group I, n: 19, M/F: 10/9) and patients with negative skin test were grouped as non-allergic asthma group (group III, n: 17, M/F: 10/7). All the patients were taking inhaled corticosteroids and were clinically asymptomatic. Patients in allergic rhinitis group were chosen among the ones who were referred to allergic diseases department (group II, n:22, M/F: 11/11) and had positive skin test. Blood samples from the patients with active rhinitis symptoms were taken one month after infection treatment. Patients who applied to Internal Medicine outpatient clinic for check-up were enrolled into the control group (group IV, n:36, M/F: 19/17). Patients with active infection finding and iron deficiency anaemia were excluded from the study. Some characteristics of the study groups are given in Table 1.
Some characteristics of the study groups.
| Patient groups | N | Sex (M/F) | Age | Smoking rate (%) |
| Group-I (Allergic asthma) | 19 | 10/9 | 40.21±12.26 | 56±10 |
| Group-II (Allergic rhinitis) | 22 | 11/11 | 29.32±9.3 | 55±11 |
| Group-III (Non-Allergic asthma) | 17 | 10/7 | 38.18±18.10 | 47±12 |
| GROUP-IV (Control) | 36 | 19/17 | 43.67±16.46 | 53±8 |
After an overnight fast, blood samples were drawn from the antecubital vein into the tube containing EDTA to measure the erythrocyte Cu-Zn SOD, erythrocyte GSH-Px activity and erythrocyte levels of MDA. Whole blood was separated into plasma and erythrocyte fractions by centrifugation (4000×g, 10minutes) at 4°C. As soon as possible after separation, the erythrocyte fractions were washed three times with saline. Then, erythrocytes were lysed with cold distilled water (1:4), stored in refrigerator at 4°C for 15minutes and then their membranes were removed by centrifugating them at 4°C for 30minutes with 20000×g. Plasma samples and erythrocyte lysate were stored at - 70°C until assay.
Erythrocyte MDA levels along with CuZn-SOD and GSH- Px activities in the erythrocyte lysates were measured by the method of Aydin14
CuZn-SOD activity in erythrocytes lysate was measured. Erythrocytes lysate samples were diluted with 10nM phosphate buffer pH 7.0 about 400 fold. Twenty-five microlitres diluted erythrocytes lysate samples were mixed with 850μL substrate solution containing 0.05 mmol / L xanthine and 0.0025 mmol / L 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyl tetrazolium chloride (INT) in a buffer solution containing 50 mmol/L N-cyclohexyl-3-aminopropanesulfonic acid (CAPS) and 0.94 mmol/L EDTA pH 10.2. Then, 125μL xanthine oxidase (80 U/L) was added to the mixture and absorbance increase was followed at 505nm for three minutes against air. Twenty-five microlitres phosphate buffer or 25μL various standard concentrations in place of sample were used as blank and standard determinations. ΔA/ minute of standard or sample was calculated. All standard and sample rates were converted into percentages of the blank rate and subtracted from 100% to yield a percentage inhibition. CuZn-SOD activity was expressed as U/gHb.
GSH- Px activity in erythrocyte lysate was also measured. Reaction mixture was tris buffer 50 mmol/ L pH 7.6 containing 1 mmol/ L of Na2EDTA, 2 mmol / L of reduced glutathione (GSH), 0.2 mmol/L of NADPH, 4 mmol/L of sodiumazide and 1000 U of glutathione reductase (GR). Fifty microlitres of plasma and 950μL of reaction mixture were mixed and incubated for five minutes at 37°C. Then, the reaction was initiated with 8.8 mmol/L H2O2 and the decrease in NADPH absorbance at 340nm was followed for three minutes. The haemoglobin concentrations of erythrocytes lysate were determined by the cyanmethaemoglobin method. Enzyme activities were reported as U/gHb in erythrocytes lysate.
Erythrocyte MDA levels were determined. After the reaction of thiobarbituric acid with MDA, the reaction product was measured spectrophotometrically at 532nm. Tetrametoxy propane solution was used as standard. MDA levels of erythrocytes were expressed as nmol/gHb, respectively.
Copper levels in the plasma samples were measured using flamed atomic absorption spectrophotometry technique. Samples were diluted with %1 HNO3. Cu calibration graph was drawn with 4, 6 and 8μg/ml standard solutions and Cu levels in the samples were measured accordingly. 324.7nm wavelength was used for copper and two measurements were done for each sample. The results are expressed as μg/ml.
Plasma zinc levels in the samples were measured with the same method. Ground correction with deuterium lamp was used. Zn calibration graph was drawn with 0.5, 1, 1.5μg/ml standards. 213.9nm wavelength was used for zinc and two measurements were done for each sample. The results are expressed as μg/ml.
All the data were combined in a common database and statistical evaluation performed. Statistical package program was used to evaluate the results for social sciences (SPSS, version 11, SPSS Inc USA). Kruskal-Wallis test was used to evaluate non-parametric data. Mann Whitney U test was used for comparison of the controls and patient groups. Confidence Interval (CI) was taken as 95%, p<0.05 values were accepted as statistically significant. Results are provided as mean±standard deviation.
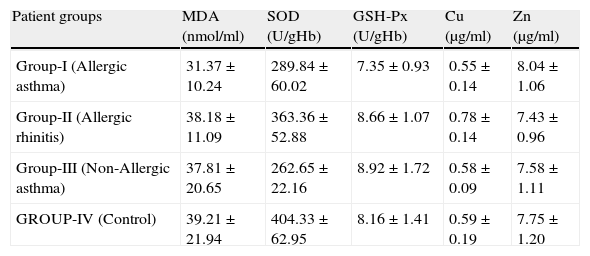
ResultsSome characteristics of patient groups and controls are shown in Table 1. Our patient groups consist of a total of 58 individuals (31 men and 27 women). Thirty-six healthy controls were also enrolled into the study. Age range was 18 to 64 years and mean ages of the groups were as in Table 1. Sex distribution had no difference (x2=2.72, p=0.099) among these groups while there were statistically significant differences between patient and control groups in terms of age (p=0.005) and smoking rates (x2=4.255, p=0.039). However, all of the patient groups had statistically significant decreases in their erythrocyte CuZnSOD enzyme activity in comparison with those of controls (p<0.001 for allergic asthmatics, p=0.008 for allergic rhinitis patients and p<0.001 for non-allergic asthmatics) (Figure 1). Similarly, erythrocyte GSH-Px enzyme activity was also not different when compared to that of the control group (p=0.057 for allergic asthmatics, p=0.145 for allergic rhinitis patients, and p=0.094 for non-allergic asthmatics). All of the patient groups had no difference from those of the controls with respect to erythrocyte MDA levels (p=0.473 for allergic asthmatics, p=0.149 for allergic rhinitis patients, and p=0.627 for non-allergic asthmatics). Only the patients with allergic rhinitis among the patient groups had statistically significant higher plasma copper levels than controls (p<0.001) (Figure 2). No statistically significant difference was established between patient groups and controls with respect to plasma zinc levels (p=0.518 for allergic asthmatics, p=0.252 for allergic rhinitis patients, and p=0.703 for non-allergic asthmatics). The comparison of anti-oxidant system parameters in all groups is shown in Table 2.
Comparison of anti-oxidant system parameters in all groups.
| Patient groups | MDA (nmol/ml) | SOD (U/gHb) | GSH-Px (U/gHb) | Cu (μg/ml) | Zn (μg/ml) |
| Group-I (Allergic asthma) | 31.37±10.24 | 289.84±60.02 | 7.35±0.93 | 0.55±0.14 | 8.04±1.06 |
| Group-II (Allergic rhinitis) | 38.18±11.09 | 363.36±52.88 | 8.66±1.07 | 0.78±0.14 | 7.43±0.96 |
| Group-III (Non-Allergic asthma) | 37.81±20.65 | 262.65±22.16 | 8.92±1.72 | 0.58±0.09 | 7.58±1.11 |
| GROUP-IV (Control) | 39.21±21.94 | 404.33±62.95 | 8.16±1.41 | 0.59±0.19 | 7.75±1.20 |
Free oxygen radicals (FOR) or free radicals or toxic oxygen metabolites occur after a chain of events formed in steps and finally leads to cellular death, tissue damage and necrosis. Basal resource of increased oxygen radicals in asthma is inflammatory cells. It is known that eosinophils are the most dominant inflammatory cells both in allergic and non-allergic asthma. Eosinophils have even greater ability of FOR synthesis than neutrophils.15 Oxidants destroy ciliary functions of respiratory epithelium and decrease surfactant activity while increasing mucus secretion, activity of cytokines and proteases, neutrophil chemotaxis and alveolar permeability and smooth muscle contractility. The relationship between bronchial hyperactivity and FOR seen in patients with asthma is revealed both in animal and human models. In one study, it was suggested that FOR increased secretion of mediator from mast cells, which is one of the key events in the beginning of allergic inflammation.16
Selenium among trace elements is of great importance for GSH-Px enzyme activity. Studies showed that GSH-Px activity decreased and antioxidant defence was weakened in the deficiency of selenium.17 In our study, GSH-Px activity was directly measured and was found to be indifferent in all patient groups compared to those of control group subjects, indicating its preserved function in our patient groups. In a double blind randomised study conducted by Hasselmark et al., it was shown that GSH-Px levels and serum selenium levels of patients with asthma who were administered selenium increased and a symptomatic healing occurred.18 In a study conducted in Australia, it was found that GSH-Px was affected by atopy, age and sex along with selenium level and a relationship between the severity of disease and decreased activity was found.19 Although it seems difficult to reach clear-cut conclusions in allergic patient groups with respect to their GSH-Px activities, our observation suggested a functioning GSH-Px enzyme in these patients.
Zinc and copper levels checked in our study are basic nutritional substances that are known as antioxidants besides taking part as cofactor in many metalloenzymes. These trace elements play a crucial role in the continuance of functions and integrity of biomembranes and in protection against free radical damage. In a diet lacking zinc and copper, antioxidant protection gets weak. Two important changes occur in lungs with the decrease in serum zinc levels; first; changes occur in cell membrane (membrane stability and integrity is destroyed, causing secretion of enzymes from lysosomes and histamine from mastocytes and therefore the membrane becomes unprotected against free radicals). Secondly, CuZnSOD structure and activity is destroyed and its ability to remove free radicals decreases. When several literature data related to plasma Cu and Zn levels in patients with bronchial asthma is reviewed, lower Zn levels and higher Cu levels than the control group are mostly reported.20,21 In our study, plasma Cu levels were not found to be significantly higher in asthmatic patients than those of the control group. Kadrabo et al. checked plasma copper and zinc levels in 22 patients with intrinsic asthma and 33 healthy controls and plasma zinc levels were found to be significantly lower than the control group,22 in contrast to our study. In a study conducted by El-Kholy et al., zinc and copper levels both in serum and hair were assessed in bronchial asthma, atopic dermatitis patients and healthy controls. In that study, zinc levels were found to be lower and copper levels were found to be higher than those of the control group, so the authors stated that zinc deficiency caused aggravations in allergic diseases and, therefore, zinc administration in these patients would lead to symptomatic healing.21 A decrease in serum copper and zinc levels causes a decrease in CuZnSOD activity, and a reduction in protection mechanisms against free oxygen radicals will occur and lung damage will increase.21 In our study, a significant reduction was found in CuZnSOD activity compared to control group in the allergic, non-allergic asthma, and allergic rhinitis groups (p<0.001 for group I and III, p=0.008 for group II). In a very recent study conducted right after a study explaining the role of zinc in protection of airway epithelium and procaspase-3 regulation,23 insufficient activity of CuZnSOD was held responsible for bronchial hyperreactivity in asthma and remodelling and it was shown that cells were gone to apoptosis.24 Zinc deficiency does not only take part in the structure of CuZnSOD but also shows an anti-apoptotic effect for respiratory epithelium. In a study conducted by Raeve et al., antioxidant response of respiratory epithelium in airway inflammation in asthma was investigated. SOD activity was found to be lower than the control group in patients who were not administered inhaled corticosteroids and CuZnSOD activity was declared as normal since inflammation decreased in patients who received inhaled corticosteroid.25 In the same study, correction of CuZnSOD activity was seen with inhaled corticosteroid treatment. It was shown that corticosteroids decreased secretion of reactive oxygen radicals from neutrophils due to their anti-inflammatory effects and increased antioxidant effect by stimulating glutathione synthesis in liver.12 A decrease in trace element levels is seen during acute asthma attacks and an increase in their levels is observed after treatment.26 In our study, all patients in the asthma group were clinically stable and taking inhaled corticosteroid treatment. In accordance with the above-mentioned studies, our results suggested a decrease in CuZnSOD activity in asthmatic patient groups.
Since direct measurement of FOR in in vivo systems is difficult, lipid peroxidation products were studied as indicators of FOR. These studies generally supported the fact that systemic oxidant stress existed in asthma; a decrease in antioxidant capacity occurred with this oxidant stress, and interestingly, this stress continued in stable asymptomatic phases of the disease.27,28 Aldehydes as lipid peroxidation products [MDA, acrolein, n-hexanal(C6) n-heptanal (C7) n-nonanal (C9), 4 hydroxynonenal (HNE) and 4 hydroxyhexenal (HHE)] have drawn tremendous attention from researchers and studies to reveal their role in asthma pathogenesis are conducted by investigating concentrations in various mediums such as induced sputum, or inspired air. In another study conducted in our country, oxidant and antioxidant situation was investigated in moderate asthma (similar to the stable group in our study) and MDA level was found to be low, SOD activity was found to be high (oxidant effect is decreased and antioxidant effect is dominant) and it was stated that oxidant and antioxidant situation was not visibly affected in the moderate asthma group at the end of study or these two parameters were not sensitive enough to show this situation.29 In a similar study, patients with nocturnal asthma were considered and MDA levels were found to be higher and GSH-Px activities were found to be lower than those of the control group.28 Another study was conducted in children, MDA levels were found to be high in the patient group but no difference was found in Zn and Cu levels. In the same study, iron (Fe) levels were found to be significantly higher in the patient group and selenium (Se) levels were found to be low. So, they stated that increased Fe and decreased Se levels could be held responsible for oxidant-antioxidant imbalance in asthma.30 In a study conducted in India, elevation of lipid peroxidation products both during asthma attack and asymptomatic stable period was found.31 High MDA levels did not return to normal despite treatment in a study investigating effects of treatment on MDA levels in asthmatic patients.32 IL-8, ECP (eosinophilic cationic protein) and MDA levels were found to be effective in monitoring airway inflammation (checked in induced sputum) in a previous study.33 Other than the studies conducted with MDA, in studies conducted with coenzyme Q10, the relationship between its low levels and oxidative stress has been found and many researches on this topic are also going to be seen.34,35 MDA elevation indicated oxidative damage in asthma and it is logical to expect increased MDA levels in acute aggravations; and it is shown that MDA levels never decrease to the levels in healthy individuals despite effective treatment, therefore, it earns the definition of “chronic inflammatory airway disease”. In our study, all of the patient groups had no difference from those of the controls with respect to erythrocyte MDA levels. This finding suggests that preserved components of anti-oxidant defence system seem to overwhelm with the oxidant insult occurring in these patient groups.
Patients with allergic rhinitis are accepted as pre-asthma because asthma can develop in most patients with allergic rhinitis. Therefore, it seems an accurate approach to suggest that oxidative stress plays a role in the pathogenesis of allergic rhinitis. A study was conducted to investigate free radical damage in patients with nasal polyps and MDA levels were found to be significantly higher than the control group in excised polyp tissues and it was considered that free radicals play a role in the development of nasal polyps.36 In reviewing the literature, we were unable to find a study in which MDA levels were investigated in patients with allergic rhinitis.
In our study, CuZnSOD activity was found to be significantly lower (p=0.008) and higher Cu levels were observed (p<0.001) in the patients with allergic rhinitis compared to those of the controls. Absence of active inflammation findings (i.e acute aggravation) in any of the groups indicated that patients in all groups were clinically stable. Therapeutic approaches to increase low CuZnSOD activity in patients with allergic rhinitis will become more popular in the near future.37 Removal of free oxygen radicals by increased CuZnSOD activity and, therefore, avoidance from damage is the aim. Similarly, new treatment strategies with CuZnSOD mimetic agents are being developed. In a previous study, trace elements were investigated in sinusitis, rhinitis and otitis and plasma zinc and copper levels were found to be significantly lower than those of the control group.38 It was found that zinc treatment added to antibiotic treatment in acute bacterial rhinosinusitis was not beneficial. Although there are many studies suggesting that zinc and copper addition with dietary changes lead to clinical healing, studies to investigate decrease of oxidative stress with zinc and copper addition to diet in patients with allergic rhinitis that lead to symptomatic healing barely exist. Zinc, vit-C and magnesium in diet were emphasised in few studies39 and also it is stated that creams including zinc can be beneficial topically40 or zinc gluconates can be beneficial when administered orally.41
Considering our data, it can be concluded that, despite their clinically stable conditions, oxidative stress exists in allergic rhinitis patients and particularly in asthma patients, suggesting its role in the pathogenesis of these disorders. The fact that low plasma CuZnSOD can be found in patient with asthma and allergic rhinitis in the absence of an increased lipid peroxidation may be attributable to well-preserved functions of other protecting scavenger enzyme activities. As the interactions among molecules of free oxygen radicals are more clearly revealed, we may have the opportunity to develop new treatment strategies including mimetics which enhance the some scavenging enzyme activities such as CuZnSOD and GSH-Px.