INTRODUCTION
The pine processionary caterpillar (Thaumetopoea pityocampa) belongs to the Thaumetopoeidae family and is one of the main European forest pests1. Approximately 150 species of lepidoptera have been described which are capable of causing harm to the human skin2-4. Several species of caterpillars are equipped with an urticant device containing chitinous spines, capable of penetrating the dermis and causing contact dermatitis known as erucisms5. The urticant capacity of its hairs or spicules is well known from antiquity, although the first descriptions correspond to Reaumur (1736) and Fabre (1900)3.
The occurrence of skin manifestations, generally located in exposed areas, has been described; they are caused mainly by a toxic-irritative mechanism1,3. These effects are usually due to an unspecified mechanism of basophil degranulation, caused by the caterpillar's urticant hairs, harpoon-shaped, capable of being airborne1 and which, upon entering the skin and breaking inside, inject histamine releasing substances5,6. The combination of physical phenomenon (skin penetration of the hairs) and chemical phenomenon (discharge of toxic substances) is accountable for the pathological symptomatology induced by the pine processionary2,7,8. A protein has been described in the processionary's spicules, Thaumetopoein5, responsible for histamine releasing phenomenon due to mast cells degranulation triggered by an IgE-independent mechanism5,6. However, clinical symptomatology, due to mainly occupational exposure, in which an immediate hypersensitivity mechanism was proved, has been published1,2,3,9,10.
The goal of this article is to show four cases of immediate hypersensitivity reaction caused by the pine processionary in patients not occupationally exposed.
MATERIAL AND METHODS
Patients
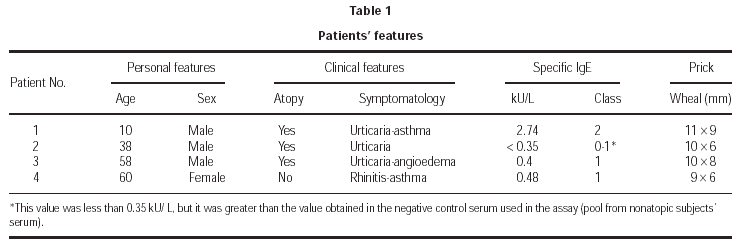
The study involved 4 patients with diverse clinical symptomatology arising from non-occupational exposure to the pine processionary caterpillar, without direct contact with the animal and with a shared past history of having spent time at large pine groves where these caterpillars abound. Clinical differences between patients are show in table I.
Case 1
10-year old male patient with a history of rhinitis-asthma due to sensitisation to pollen and dog epithelium, and of gastrointestinal allergy due to apples. The patient reported, during spring and whenever he played at a field with pine-trees full of processionaries, episodes of generalised urticaria together with, on occasions, sibilant dyspnoea. Apart from these isolated incidents, the patient did not show episodes of breathing difficulty.
Case 2
38-year male, no family history of allergy, with a history of acute urticaria due to sensitisation to Anisakis simplex. The patient recently reported suffering from pruriginous wheal-like lesions in exposed zones when trimming cypress trees, which he attributed to the processionary caterpillar that parasitizes the pine trees located in the surroundings of this Cupressaceae, since in the absence of the processionaries the patient showed no clinical symptoms in contact with cypress.
Case 3
A 58-year old male patient without a personal or family history of atopy came to consultation after suffering from pruriginous wheal-like lesions in legs, arms and neck, fever symptoms and palpebral angioedema as a result of having touched the soil surrounding a marsh which he visited every weekend, and located in an area with an abundance of pine-forest parasitized by the processionaries. Symptoms appeared from February to May, although they are more frequent during February and March. The symptoms became less intense in an urban area (the city of Madrid in Spain), where the patient usually lives. None of these symptoms appeared during the rest of the year nor symptoms of rhinoconjunctivitis nor bronchospasm.
Case 4
A 60-year old female patient with a family history of atopy (pollinic daughter) came to consultation because, for the last three years, she had been suffering during the spring season (March and April), wheal-like lesions together with palpebral angioedema, as well as rhinitis, dry coughs and sibilant dyspnoea, during last year.
The patient reported how her symptoms worsened in the open air, remaining without symptoms out of the referred season. She did not show sensitivity to weather changes either. Her environment is hygienic, without the presence of animals. She lives in the countryside (province of Avila, centre of Spain) with abundant cypress and pine trees parasitized by processionary caterpillars, to which the patient attributes her symptoms. Her health improves whenever she left the countryside for the urban environment of Madrid. During the symptomatic episodes she received treatment with ebastine, budesonide and bronchodilators on demand with a positive evolution and symptomatic control.
None of the patients associated the skin symptoms with drugs, food or physical factors.
Preparation of the extract
Thaumetopoea pityocampa specimens in L5 larvae stage were ground in a pool of liquid nitrogen into a course "powder" of frozen fragments in a mortar and extracted by magnetic stirring in agitation in 50 mM phosphate-buffered saline (PBS) at pH 7.5 during 4 h at room temperature. After centrifugation, supernatant was dialyzed against water. The dialyzed extract was filtered through a 0.22 μm-pore diameter membrane and freeze-dried.
Skin testing
Skin prick tests (SPTs) were carried out with different aeroallergens (pollens, animals epithelia, moulds, mites and cockroaches) and Anisakis simplex extracts and with an extract of caterpillars at the last larval stage (L5), provided by Laboratorios Bial-Arístegui. The extract was tested on control subjects (atopic and non-atopic). An immediate reading was taken after 15 minutes, which was considered as a positive SPT when the size of the wheal was equal or less than 3 mm with respect to the control with saline serum.
Complementary clinical tests
All patients underwent a basic physical exam which included hemogram with leucocite formula and VSG, biochemistry, chest X-rays, identification of parasites in faeces and hydatidic serology.
Determination of specific IgE
The level of specific IgE to usual aeroallergens, Anisakis simplex, Ascaris and Echinococcus was measured by CAP (Pharmacia Diagnostics, Uppsala, Sweden). Measurement of specific IgE to the caterpillar extract was performed by using Bial-Arístegui discs with the allergen coupled (10 mg/mL). Cellulose discs were activated with BrCN by using the method of Ceska & colleagues11 and development was carried out with the HY·TEC EIA Kit for specific IgE (HYCOR Biomedical Ltd., UK).
SDS-PAGE Immunoblotting
SDS-PAGE was carried out according to the method of Laemmli12, 12.5 % and 4 % of acrylamide were used for separating and stacking gel respectively. To prepare the samples under reducing conditions, they were dissolved in 0.125 M HCL-Tris, pH 6.8 and were dissociated with 0.1 % SDS and 5 % β-mercaptoethanol at 100 °C for 5 minutes. For non-reducing conditions the β-mercaptoethanol, was omitted in the sample buffer. 20 μg protein, according to Bradford (13), were applied per lane.
After electrophoresis, gels were stained by diffusion in 0.1 % Coomassie Brilliant Blue R-250 dissolved in methanol/acetic acid/distilled water (4:1:5). Separated proteins bands were electrophoretically transferred to polyvinylene difluoride (PVDF) essentially described by Towin et al14 and blocked for 1 h at room temperature with 0.05 % Tween-20 in Tris-buffered saline (TBS). Membranes were incubated overnight at 4 °C with patient's serum followed by antihuman IgE-horseradish peroxidase conjugate incubation and detected by the chemiluminescence method as recommended by the manufacturer (ECL-Plus; Amersham Pharmacia Biotech).
Respiratory functional exploration and bronchial challenge test
In patient 4, the following tests were made: spirometry and volume/flow curves, bronchial hyperreactivity test with histamine (Cockcroft method)15 and specific bronchial challenge test, carried out using Chai's accumulative method16 with the pine processionary antigen.
RESULTS
Skin testing
The results against the extract of pine processionary are shown in table I. Skin tests carried out on atopic and non-atopic controls were negative.
All patients, except n.º 4, showed positive SPT results against grasses and cypress pollens and dog and cat epithelia extracts. Also, positive SPT results were obtained against apples extract in Case 1 and against Anisakis simplex extract in Case 2. In all patients the tests yielded negative results against mite, mould and cockroach extracts.
Determination of Total and Specific IgE
Determination of total IgE was 639 kU/L in Case 1, 98.2 kU/L in Case 2 y 103 kU/L in Case 3. Specific IgE against the pine processionary was positive in 3 sera of the four patients (table I).
The levels of specific IgE against pollens were positive (Classes 3-4) in the first 3 cases. The determination of specific IgE against animal epithelia was positive in the second patient.
Western blotting
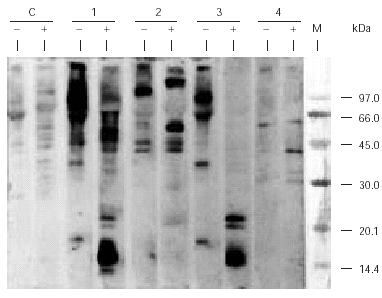
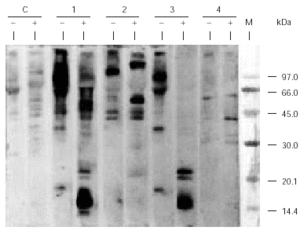
SDS-PAGE Immunoblotting carried out with the caterpillar extract and incubating the sample with patient sera showed the existence of several IgE-binding bands of molecular masses ranging between 14 and 107 kDa whereas no reactive bands were detected with control serum. A similar IgE-binding band pattern was obtained when the sample was incubated with patient 1 or patient 3 serum. The apparent molecular mass of the reactive bands which appeared in this pattern depends on the conditions of electrophoresis. In absence of β -mercaptoethanol (non reducing conditions), bands of 90, 64, 36, 18, 57, and 107 kDa were detected whereas in presence of such agent, the molecular mass of the bands were: 60.5, 40.6, 55, 22, 20.5, 16/15.6/15, and 14 kDa (fig. 1). This fact shows the importance that disulphur bridges have for the maintenance of the tertiary and/or quaternary structure of these proteins.
Figure 1.--SDS-PAGE Immunoblotting of the pine processionary extract incubated with the patients' serum. C: control serum (pool from nonatopic subjects' serum); 1: Patient 1 serum; 2: Patient 2 serum; 3: Patient 3 serum; 4: Patient 4 serum. Lane (-): Samples without β -mercaptoethanol (non-reducing conditions) Lane (+): Samples with β-mercaptoethanol (reducing conditions) M: Molecular mass marker.
Respiratory functional exploration of Patient No. 4
Both the spirometry and the flow-volume curves were found to yield results within the limits of the reference range.
The bronchial hyperreactivity test with histamine carried out in September, symptomatic season, produced negative results.
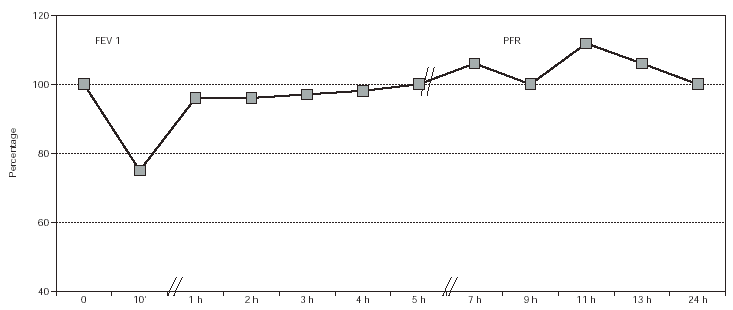
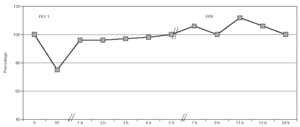
In the specific bronchial challenge test with pine processionary antigen an isolated immediate response was obtained, with a decrease in FEV1 of 24 % at a concentration of 10 mg/mL (fig. 2).
Figure 2.--Bronchial challenge test with Pine Processionary extract. Patient No. 4.
DISCUSSION
The pine processionary is the common name for the caterpillar of the nocturnal lepidoptera Thaumetopoea pityocampa17,18. Its distribution is typically Mediterranean, and in Spain it can be found mainly in the Central and Southern areas of the Iberian peninsula17. In general, its location is conditioned by the weather, and it could parasitize local as well as foreign pine species17.
Its urticant hairs have been described to cause harmful effects in humans, especially on the skin and the eyes, although also bronchial effects and anaphylactic shock have been occasionally reported1,2,19,20,21. These spicules, with an approximate length of 150-200 μm and a diameter of 5 μm, are detectable in air using techniques designed for airborne micro-organisms and pollen research and may be able to penetrate the human respiratory system as far as the trachea and primary bronchi, thus provoking respiratory pathology7,18. The amount of spicules found is directly related to the existing distance to the production zone, local meteorological conditions, and the processionary's biological cycle18.
Over the last few years several cases have been reported1,2,9,16 which show the existence of immediate hypersensitivity mechanisms through SPT, determination of specific IgE and SDS-PAGE Immunoblotting. Werno et al, in 1993, while working with pine processionary extracts detected in Western blot the presence of IgE binding bands of 28 kDa (which they named thaumetopoein) and a greater band of 45 kDa9. Although the results of Immunoblotting achieved in our study do not show a 28 kDa band, they do show an intense IgE binding zone ranging from 13-15 kDa, detected with serum of patient 1 and patient 3, whose molecular mass matches with that obtained, by means of western blotting, by Vega et al for the most prevalent allergen when the serum of 16 patients who suffered from contact urticaria were used10. This reactive zone, as shown on figure 1, only appears in the blot, when the sample was treated with a reductive-disrupting agent of disulphur bridges, like β -mercaptoethanol. So, if Western blot is carried out in the absence of β-mercaptoethanol, bands with molecular mass of 90, 64, 36 and 18 kDa are detected, whereas if it is carried out in the presence of β-mercaptoethanol, bands of 55, 22, 20.5, 16/15.6/15 and 14 kDa appeared. Therefore, the 14 kDa protein probably belongs to a larger protein structure whose native structure is maintained by the presence of disulphur bridges. In this context it must be noted that thaumeopoein has been described as a 28 kDa protein which produces two polypeptides of 13 and 15 kDa respectively in denaturalising electrophoretic conditions (SDS and β-mercaptoethanol)5. All of these results let us to propose that the thaumetopoein, apart from the aforementioned toxic effects, could be acting as an allergen, generating an IgE-mediated hypersensitivity response. These results show a contrast with those described by Alamar et al and Moneo et al20, in which no changes were observed in electrophoretic mobility due to the action of reductive agents.
In a former study, Vega et al2 obtained inconsistent results in evaluating specific IgE levels to pine processionary extracts by means of ELISA. However, the EAST method, used in this study, has allowed us to detect significant levels in the patients' serum (class 1 and 2). This fact may be attributed either to the better binding of the involved allergens to the solid phase we used in EAST (cellulose discs) than the one they used in ELISA or, alternatively, because the weight percentage of the involved allergens encountered in the processionry extract used by Vega et al were much lower than the value found in ours.
The four cases here presented share some common features: having been in pine-groves several hours before the allergic reaction without direct contact with the caterpillars, non-occupational exposure, the presence of symptoms during the months of February and April, and skin involvement in the form of urticaria-like eruptions. Vega et al noted that 37 % of patients exposed as a result of their occupation presented symptoms from October to December (larvae stage of development L3 and L4), while in patients non occupationaly exposed symptoms appeared in springtime (larvae phase L5)2 Although the occurrence of symptoms in the patients here studied took place in springtime (typical for non-occupational allergy), patients 1 and 4 suffered from symptoms such as rhirnorrea and dyspnoea only described by Vega et al for those patients with high exposure because of occupational reasons2. It is worth pointing out that the severity of the clinical symptoms shown by patients was diverse: from a simple urticarial eruption as a sole symptom in Case no. 2, to urticaria and angioedema (Case no. 3), through to cases of bronchial asthma in Cases no. 1 and 4. Also, in this work it is worth noting the range of starting ages for the occurrence of symptoms, from a child, who, to the best of our knowledge, represents the second case of child processionary allergy described, to adult patients. The age interval described by Vega et al in their series of patients with contact urticaria 19 ranges from 4 to 73 years. The atopic character of 3 of (our patients) the patients here studied matches the high percentage of atopic patients (62 %) found among non-occupational patients by others authors2.
Therefore, airborne urticating hairs of T. pityocampa should be considered, in areas close to pine groves, as seasonal inhalant allergens capable of causing allergic pathologies in patients without the requirement of occupational exposure. This fact is specially significant for countries like Spain, where this lepidoptera constitutes an important forest pest.