The role of infections on the development of atopy is still widely debated. We aimed to evaluate the effects of neonatal severe sepsis and consequent antibiotic treatment on the development of atopy and allergic diseases.
Material and methodsA retrospective enrolment at school age of children with a clear history of neonatal sepsis (NS) was performed from registers of neonatal intensive care units. A normal control was assigned to each patient with sepsis. Thirty six cases with sepsis (18 males, 18 females) and 36 controls (21 males, 15 females) were selected (8.5±3.6 yrs). Physical examination and lung function evaluation were performed. Atopic status was verified by blood eosinophil count, total IgE serum level and skin prick tests (SPT).
ResultsSPT positivity for at least one allergen was present in 30% of subjects in both groups. No difference for all investigated parameters between groups and no influence by other factors such as familiarity or gender was observed. No correlation was associated to NS history.
ConclusionsNeonatal sepsis, even if clinically severe and dramatic, could represent an event too limited and really precocious in life to influence the development of immune response. Furthermore, other factors, besides infections, may influence the atopic future of newborns.
Asthma and allergy inflammatory responses are associated with immune shift toward Th2 phenotype with consequent over-production of Th2-associated cytokines and inflammatory mediators.
According to the hygiene hypothesis, an early infection during childhood could downregulate immunity and suppress the development of allergic diseases.1,2 The Th1/Th2 balance could be related with influence/exposure during early childhood and infancy to allergens and infections. This process could start during the intra-uterine period.3 The prenatal and early childhood are considered particularly crucial in developing and maintaining a normal balance Th1/Th2. There are also other factors, like domestic animals, which are related to the decreased risk of atopic disease.4 However, the post-birth period is considered particularly important.5
Several previous studies showed an inverse correlation between asthma and the overall burden of infections even if the role of infections on the prevention/suppression of later development of atopy and allergic diseases is currently widely debated.6,7 Furthermore, the influence of the use of antibiotics in the first year of life is controversial. Some studies reported an association8 between recurrent antibiotic use and atopic disorder, while another did not support this hypothesis.9 However, it has been demonstrated in an experimental model that antibiotic use during infancy may disturb the intestinal microflora and prevent postnatal Th1 cell maturation, thus resulting in a Th2-polarised immune deviation.10
Moreover, it has yet to be clearly defined what type of infectious disease is a protective factor in the development of atopy, i.e. if the number; the severity or the type of infection (gastrointestinal or respiratory); the kind of infectious agent; the time exposure; or the combination of these factors could be decisive.4
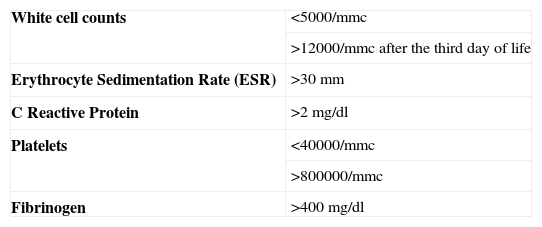
MethodsPatients’ retrospective enrolment was performed from the registers of two neonatal intensive care units, in the paediatric departments of Verona and Bolzano Hospitals. In order to study patients able to perform lung function tests, children aged over five years and with a definite history and clinical diagnosis of NS were selected. They were all born at least at 34 weeks of gestational age, and thus influences related to severe prematurity can be excluded. They all presented positive blood cultures with bacteria other than Staphylococcus epidermidis and at least two of the laboratory parameters shown in Table 1. Subjects were treated with a double antibiotic therapy: most of them received ampicillin and netilmicin combined treatment. Antibiotics were continued for at least ten days according to internal protocols. A normal control was assigned to each patient with NS. As control we considered the newborn consecutively registered in the nursery of the same hospitals.
A validated questionnaire, the International Study of Asthma and Allergies in Childhood (ISAAC) written questionnaire (WQ)11 was used to evaluate the past and current presence of asthma, rhinitis and atopic dermatitis. Furthermore, physical examination was performed to assess the presence of signs and symptoms of atopic dermatitis, wheezing or rhinitis.
Atopic status was verified by blood eosinophil count, total IgE serum level and SPT. Standardised extracts for foods (egg, milk), pollens relevant to the region (Grass mix, Parietaria judaica, trees), house dust mites (Dermatophagoides pteronyssinus and farinae), animal dander (cat, dog), alternaria (ALK-Abello’, Milan, Italy), as well as a saline negative control and a histamine control (10mg/ml) were used.
Lung function tests were obtained using a Pulmograph spirometer; evaluations were performed both at baseline and after bronchodilator administration.
The protocol was approved by the Local Ethical Committee and informed consent was obtained by children and parents.
StatisticsPaired Student's t test was used to evaluate differences between subjects and controls. Pearson's correlation test was used to study the correlation between presence of NS and results of the different end points. We have considered the level of significance when p<0.05.
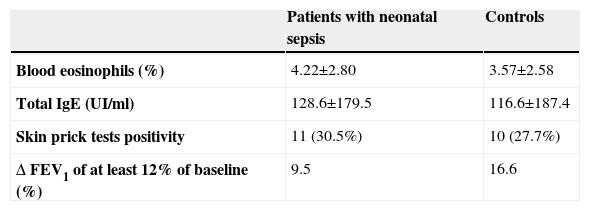
ResultsThirty six cases with NS (18 males and 18 females) and 36 respective controls (21 males and 15 females) were selected. Mean age of the subjects was 8.5±3.6 yrs both in the NS and in the control group. Family history of atopic diseases did not differ in the subjects (32%) and in the controls (27%). Results are given as mean +/− standard deviation and summarised in Table 2. There was a trend for more circulating eosinophils and total serum IgE in the NS group, which failed to reach a statistical significance. No significant difference for all the investigated parameters was observed between the two groups and no significant correlation was associated to NS history. A significant improvement of FEV1 after bronchodilator (>12%) was observed in 9.5% in the NS group and in 16.6% of the control group (p=n.s.).
DiscussionChildren participating in the present study suffered a severe NS early in life as documented by records of inflammatory parameters and microbiological findings.
The pathogen of NS is the first stimulus that leads to the first anti-infective immune response in childhood.4
Criteria for the diagnosis of serious bacterial infections in childhood usually present a marked heterogeneity. At present there are insufficient data to support the use of single sample selection and cut-off level as a potential marker of NS, since diagnostic sensitivity is low.12
Diagnosis of suspected neonatal infection (clinical sepsis) requires: an abnormal leukocyte counts and an increase of c-reactive protein (CRP) and at least three of the following symptoms: (i) cardiovascular: tachycardia or bradycardia, or hypoperfusion, shock; (ii) temperature instability; (iii) respiratory distress, grunting, intercostal retractions, apnoea, cyanosis; (iv) neurologic: hypotonia, lethargy; (v) gastrointestinal: intolerance to food, the residual stomach.13
However, in our study positive blood cultures were associated to laboratory parameters. Furthermore, infections were severe enough to require, in all patients, intensive care treatment, systemic antibiotic administration and in some cases (30%) ventilatory support. Therefore, there is no doubt of NS in these cases. No difference in occurrence of atopy and wheezing symptoms was observed in the NS group in comparison to control group at a mean age of 8 years. SPT positivity to at least one allergen was present in 30% of subjects in both groups. This is in agreement with the prevalence of SPT positivity recently observed in a large number of pre-school children living in the same area.14
Some studies have shown a positive correlation between viral infections, particularly if recurrent, in early infancy and subsequent development of atopy and asthma.15 Other studies failed to demonstrate a positive correlation, being subjects with at least two episodes of upper airway infections less at risk to present wheezing at age seven.16
As reported by Kopp and al., there was no significant difference in the risk of developing atopic dermatitis and allergic rhinitis in patients suffering from sepsis.4 However, a family history of atopy was a risk factor for the development of atopic dermatitis, which remains the most important risk factor in children. They also found a correlation between tobacco exposure and wheezing, cough and exacerbations in asthma.17,18
Probably recurrent or persistent infections could act in a persistent and efficacious way to drive the immune response even though reverse causation has been suggested as another explanation, i.e. an impaired type 1 immunity could be a common genetic risk factor for both the development of infections and asthma.19 Despite the limited number of cases of our study, we believe that NS could represent a characteristic model to study the role of early infections associated with very precocious antibiotic administration. Indeed, NS may lead to an early influence of immune system and the precocious use of wide spectrum antibiotics may considerably alter the intestinal microflora of the neonate.
Use of antibiotics is merely one factor in a more complex interaction of the environmental conditions in a hospital and the affected individual.
Despite this, neonatal sepsis, even if clinically severe and dramatic, did not influence the development of atopy and allergic diseases in our study. It is possible that NS could represent an event too limited and really precocious in life to have a real role in influencing the development of the immune response. Other types of infections which are more prolonged and recurrent could have a more relevant role in the development of atopy and allergic diseases. Alternatively, other environmental factors besides infections may play a stronger effect towards the development of atopy in susceptible individuals.
Finally, there is no data in the literature that have demonstrated a long-term outcome in children with neonatal sepsis with regard to atopic disease.
Conflict of interestThe authors have no conflict of interest to declare.