Prevalence of respiratory allergic diseases has increased worldwide. Identification of the aeroallergens involved in allergenic sensitisation is important for diagnosis, treatment and prevention.
ObjectiveTo verify the molecular pattern of sensitisation to aeroallergens in patients with allergic respiratory diseases using microarray technique for specific IgE antibody detection.
MethodsCross-sectional study of 101 children with allergic rhinitis was followed in an outpatient clinic. All patients had positive skin prick tests (SPT) to at least one of the following antigens: Dermatophagoides pteronyssinus, Blomia tropicalis, Blattella germanica, Lolium multiflorum, and dog and cat epithelium. Serum specific IgE antibodies (sIgE) to mites, animal epithelia, fungi, cockroach and pollens components were determined by ImmunoCAP ISAC.
ResultssIgE to group 1 and 2 mite allergens showed higher positive rates: Der p 1 (74.2%), Der p 2 (73.3%), Der f 1 (74.2%), Der f 2 (72.3%). sIgE to animal epithelia were less frequent, Can f 1, Can f 2, Can f 3 in 4.9%, 2.9%, 1.9% respectively and Fel d 1, Fel d 2, Fel d 4 in 16.8%, 0.9% and 1.9%. respectively. Sensitisation to fungi and cockroach were rare, except for Bla g 7, to which 16.8% were positive. There was no significant recognition for tree pollens group. For grass, sIgE were detected to Cyn d 1 in 16.8%, Phl p 1 and Phl p 4 in 14.8% and 12.9%, respectively.
ConclusionKnowing that the pattern of allergic sensitisation varies according to environment and population, our results reinforce the need for local studies, using molecular-based diagnosis.
Epidemiological studies have documented an increase in the prevalence of respiratory allergic diseases in different areas of the world.1–3 In Brazil, the prevalence of asthma and allergic rhinitis has similarly increased in recent years.4 Identification of the sensitisation profile to aeroallergens is essential for the diagnosis, treatment and prevention of these diseases.5
Among the methods available to define allergic sensitisation are skin prick tests (SPT), which detect in vivo the presence of sIgE antibodies and represent the most used diagnostic tool in the field of allergy.6 They have high sensitivity and specificity, in addition to results that correlate well with in vitro IgE antibody determinations.7,8
Allergic sensitisation can also be verified by sIgE measurement in serum. Advances in technology have provided new laboratory methods, in which the allergen to be tested is covalently linked to a solid phase. This confers specificity to the assay. In vitro tests offer numerous advantages such as precise quantification, lack of drug interference, safety and long-term storage of specimens.7
The allergenic extracts normally used in vivo and in vitro diagnosis come from defined sources and different extraction procedures. During this process some relevant allergenic proteins can be lost, whereas others can contaminate the extract as they were present in the starting raw material.5 Although efforts are made to control allergenic extract composition, they are still a mixture of allergenic and non-allergenic compounds.9
Recently, new biochemical methods allowed identification of allergenic molecules, the actual primary sensitising agents and triggers of symptoms. Their characterisation has been improved with molecular biology techniques.9 The antigens used for sIgE determination in microarray system (ISAC® – Immuno Solid-phase Allergen Chip, Thermo Fisher, Uppsala, Sweden) are highly purified molecules, obtained by recombinant DNA methods or natural sources. Allergenic molecules facilitate standardisation and increase the specificity of the test.5 In contrast to other conventional methods, allergen microarrays allow multiple sensitisation diagnosis in a single assay, with a minimum amount of serum.5,10,11 In addition, this technique increases the capacity for accurately identifying the allergen components associated with clinical manifestations and may help distinguish true multiple sensitisations from cross-reactions.12
In the present study, we mapped the pattern of sensitisation to inhalant components in patients with allergic rhinitis using the ISAC system for molecular-based sIgE detection.
MethodsThis cross-sectional study involved outpatients with allergic rhinitis and/or asthma diagnosed respectively according to ARIA (Allergic Rhinitis and its Impact on Asthma)13 and GINA (Global Initiative for Asthma)14 guidelines. Patients were recruited at the Pediatric Allergy Division, University Hospital, in Curitiba, Southern Brazil.
We included 101 participants (a sample that was limited by the costs of performing the microarray) with 62 males, mean age 10.7 years, and range 6–15 years, who presented positive skin reaction to at least one of the following common aeroallergens: mites Dermatophagoides pteronyssinus and Blomia tropicalis, cockroach Blattella germanica, pollen Lolium multiflorum, and dog and cat dander antigens (IPI-ASAC Brasil®). For skin prick test, a 27mm needle was introduced through a drop of allergen extract after wiping the forearm's skin with alcohol. A new disposable needle was used for each allergen. Positive (histamine 10mg/mL) and negative (glycerinated saline) controls were also carried out.15,16 After 15min, the mean wheal diameter was measured and considered positive when greater than 3mm.
To assess the molecular sensitisation pattern of the 101 patients, peripheral blood was collected by venipuncture. Serum samples for sIgE measurement were stored at −80°C until assayed in Rome, Italy, by ISAC®, a microarray technique for multiplex IgE detection based on allergenic molecules,9 in which the proteins (purified recombinant or natural allergens) were immobilised in a solid phase and 20μL of serum were incubated with these proteins under standardised conditions. The antibodies present in the serum were captured by the different allergens and following a washing step to eliminate unbound substances, the antibodies were detected by means of a secondary fluorescent-labelled anti-isotype antibody that was detected by a laser scanner. Results were considered positive if values were greater than 0.3ISU (ISAC standardised units).11,17 Specific IgE levels were reported as median (range) (Table 2).
We tested 103 allergens coming from natural sources or produced as recombinants: mites, food, animal epithelia, moulds, insects, latex, parasite (Anisakis simplex), tree and grass pollen. This study included mostly inhalant allergen components from house dust mites: Der f 1, Der f 2 (Dermatophagoides farinae); Der p 1, Der p 2, Der p 10 (Dermatophagoides pteronyssinus); Eur m 2 (Euroglyphus maynei); animal epithelia: Can f 1, Can f 2, Can f 3 (Canis familiaris – dog); Fel d 1, Fel d 2, Fel d 4 (Felis domesticus – cat); insect: Bla g 1, Bla g 2, Bla g 4, Bla g 5, Bla g 7 (Blattella germanica – cockroach); moulds: Asp f 1, Asp f 2, Asp f 3, Asp f 4, Asp f 6 (Aspergillus fumigatus); Cla h 8 (Cladosporium herbarum); tree and grass pollen: Amb a 1 (Ambrosia artemisiifolia); Art v 1, Art v 3 (Artemisia vulgaris); Bet v 1, Bet v 2, Bet v 4 (Betula verrucosa); Cup a 1 (Cupressus arizonica); Cyn d 1 (Cynodon dactylon); Ole e 1, Ole e 2 (Olea europaea); Par j 2 (Parietaria judaica); Phl p 1, Phl p 2, Phl p 4, Phl p 5, Phl p 6, Phl p 7, Phl p 11, Phl p 12 (Phleum pratense); Pla a 1, Pla a 2 (Platanus acerifolia).
Descriptive statistical analysis was performed, using Statistica (Statsoft®, Tulsa, USA) program. This study was approved by the Ethics Committee of Clinical Hospital, Federal University of Parana and all participants provided written informed consent.
ResultsAllergic rhinitis was diagnosed in 101 patients, 42.6% were classified as mild and 57.4% moderate/severe depending on the severity of the symptoms. The majority had asthma (89.1%), of which 47.5% were classified as intermittent or mild persistent, 33.7% moderate and 7.9% severe. Allergic conjunctivitis was diagnosed in 67.3% of the subjects and atopic dermatitis in 9.9%.
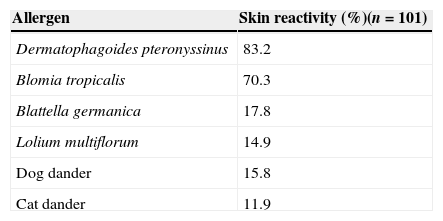
SPT identified mites as the major allergen source. Eighty-three per cent of the patients showed positive skin reaction to Dermatophagoides pteronyssinus and 70.3% to Blomia tropicalis, the other sensitisation rates were as follows: Blattella germanica, 17.8%; Lolium multiflorum, 14.9%; dog and cat epithelia, 15.8% and 11.9%, respectively (Table 1).
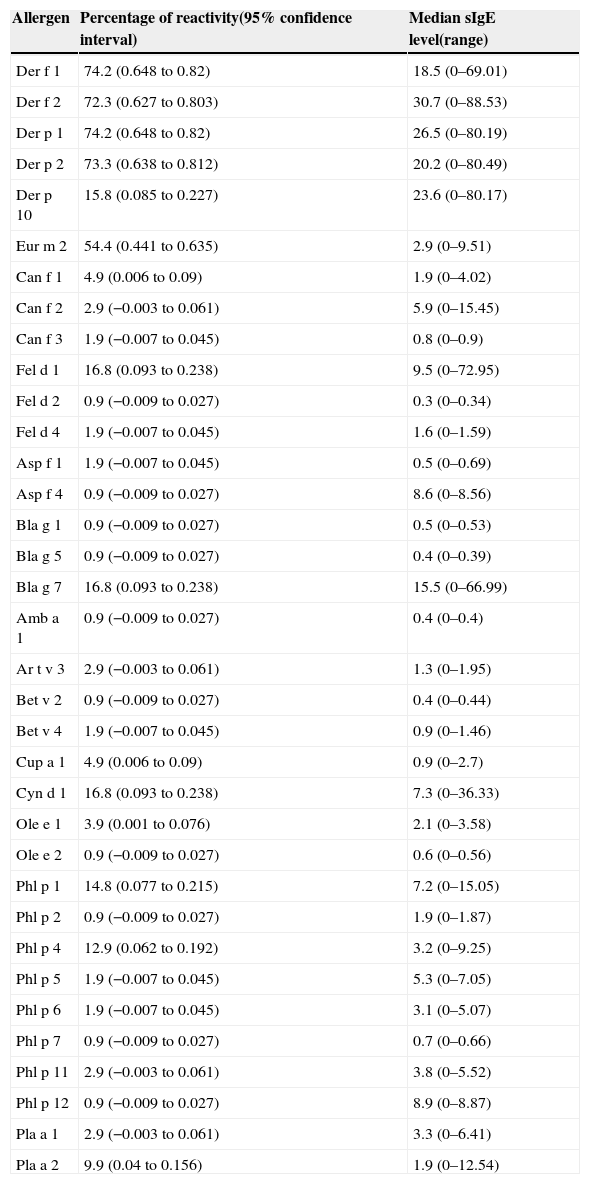
Results from serological analysis for the presence of sIgE using ISAC system were concordant with SPT results. House dust mites represented the most frequently recognised allergen source and both species Dermatophagoides pteronyssinus and Dermatophagoides farinae were equally important. In addition, Group 1 and Group 2 had similar rates of positivity: Der p 1 (74.2%), Der p 2 (73.3%); Der f 1 (74.2%), and Der f 2 (72.3%). Seventy-one (70.3%) were positive to the four Dermatophagoides allergens simultaneously. Specific IgE to a third mite species Euroglyphus maynei, was less frequently detected Eur m 2 (54.4%), all of them presented positive tests to Der p 1, Der p 2, Der f 1 and Der f 2. The highest median levels of IgE antibodies were also detected in sera from patients sensitised to mites: Der p 1: 26.5; Der p 2: 20.2; Der f 1: 18.5; Der f 2: 30.7. Frequency of sensitisation to animal epithelia, moulds and cockroaches is shown in Table 2. Sensitisation to tree pollen was low as compared to other allergens, ranging from 0 (Bet v 1) to 9.9% (Pla a 2). For the grass pollen group, IgE reactivity was higher to Cyn d 1 (16.8%) and to Phl p 12 (14.8%) (Table 2). Fourteen (13.9%) patients were both positive to Bla g 7 and Der p 10. These last two allergens are considered tropomyosins, a specific group of proteins that also includes Ani s 3 (Anisakis simplex) and Pen a 1, both positive in the same 14 subjects.
Frequency of sensitisation in 101 children and adolescents determined by microarray (ISAC system) and median sIgE levels of each allergen.
| Allergen | Percentage of reactivity(95% confidence interval) | Median sIgE level(range) |
|---|---|---|
| Der f 1 | 74.2 (0.648 to 0.82) | 18.5 (0–69.01) |
| Der f 2 | 72.3 (0.627 to 0.803) | 30.7 (0–88.53) |
| Der p 1 | 74.2 (0.648 to 0.82) | 26.5 (0–80.19) |
| Der p 2 | 73.3 (0.638 to 0.812) | 20.2 (0–80.49) |
| Der p 10 | 15.8 (0.085 to 0.227) | 23.6 (0–80.17) |
| Eur m 2 | 54.4 (0.441 to 0.635) | 2.9 (0–9.51) |
| Can f 1 | 4.9 (0.006 to 0.09) | 1.9 (0–4.02) |
| Can f 2 | 2.9 (−0.003 to 0.061) | 5.9 (0–15.45) |
| Can f 3 | 1.9 (−0.007 to 0.045) | 0.8 (0–0.9) |
| Fel d 1 | 16.8 (0.093 to 0.238) | 9.5 (0–72.95) |
| Fel d 2 | 0.9 (−0.009 to 0.027) | 0.3 (0–0.34) |
| Fel d 4 | 1.9 (−0.007 to 0.045) | 1.6 (0–1.59) |
| Asp f 1 | 1.9 (−0.007 to 0.045) | 0.5 (0–0.69) |
| Asp f 4 | 0.9 (−0.009 to 0.027) | 8.6 (0–8.56) |
| Bla g 1 | 0.9 (−0.009 to 0.027) | 0.5 (0–0.53) |
| Bla g 5 | 0.9 (−0.009 to 0.027) | 0.4 (0–0.39) |
| Bla g 7 | 16.8 (0.093 to 0.238) | 15.5 (0–66.99) |
| Amb a 1 | 0.9 (−0.009 to 0.027) | 0.4 (0–0.4) |
| Ar t v 3 | 2.9 (−0.003 to 0.061) | 1.3 (0–1.95) |
| Bet v 2 | 0.9 (−0.009 to 0.027) | 0.4 (0–0.44) |
| Bet v 4 | 1.9 (−0.007 to 0.045) | 0.9 (0–1.46) |
| Cup a 1 | 4.9 (0.006 to 0.09) | 0.9 (0–2.7) |
| Cyn d 1 | 16.8 (0.093 to 0.238) | 7.3 (0–36.33) |
| Ole e 1 | 3.9 (0.001 to 0.076) | 2.1 (0–3.58) |
| Ole e 2 | 0.9 (−0.009 to 0.027) | 0.6 (0–0.56) |
| Phl p 1 | 14.8 (0.077 to 0.215) | 7.2 (0–15.05) |
| Phl p 2 | 0.9 (−0.009 to 0.027) | 1.9 (0–1.87) |
| Phl p 4 | 12.9 (0.062 to 0.192) | 3.2 (0–9.25) |
| Phl p 5 | 1.9 (−0.007 to 0.045) | 5.3 (0–7.05) |
| Phl p 6 | 1.9 (−0.007 to 0.045) | 3.1 (0–5.07) |
| Phl p 7 | 0.9 (−0.009 to 0.027) | 0.7 (0–0.66) |
| Phl p 11 | 2.9 (−0.003 to 0.061) | 3.8 (0–5.52) |
| Phl p 12 | 0.9 (−0.009 to 0.027) | 8.9 (0–8.87) |
| Pla a 1 | 2.9 (−0.003 to 0.061) | 3.3 (0–6.41) |
| Pla a 2 | 9.9 (0.04 to 0.156) | 1.9 (0–12.54) |
Twenty-seven patients (26.7%) were sensitised to at least one food allergen analysed by the ISAC panel, nevertheless the sensitisation levels were low. Among the 42 food components, 20 (47.6%) were positive in at least one of the subjects. Shrimp and peach presented the highest frequency of IgE reactivity (respectively 15.8% Pen a 1 and 5.9% Pru p 3).
The other allergen components were not significantly detected. Ten patients were negative to all allergens tested.
DiscussionThis is the first report of the allergy profile of patients with persistent allergic rhinitis and/or asthma by the ISAC method in Curitiba, a city located in Southern Brazil. Initial screening of allergic patients by skin prick testing with six different allergen extracts revealed that house dust mites had the highest sensitisation rate. An in-depth in vitro serological analysis of sIgE antibodies using microarray technique for molecular-based diagnosis also identified mites as the most important allergen source, confirming the results obtained by SPT.
Reactivity to SPT was similar to that obtained previously in the same university allergy clinic in patients with asthma, 80% of them had positive SPT for Dermatophagoides pteronyssinus. The highest frequency of positivity was to the genus Blomia (91.3%), an important mite allergen source in tropical countries.18 Nevertheless, their allergens were not present in ImmunoCAP ISAC panel version 103, but Blo t 5 has been added to the last ISAC version, bearing 112 allergens.
Virtually all Dermatophagoides pteronyssinus reactive sera also reacted to Dermatophagoides farinae and most reacted to the storage mite Euroglyphus maynei, perhaps due to cross-reactivity.19,20 In our population, 72/75 patients positive to Der p 1 were likewise positive to Der p 2, Der f 1 and Der f 2, a finding common in many other places worldwide. More than 80% of mite allergic patients have IgE antibodies to Der p 1 and Der p 2.21 On the other hand, there were no monosensitised patients to Eur m 2, all were also positive to Dermatophagoides group 1 and 2.
The allergen profile of allergic patients in Zimbabwe was compared to an Austrian population by analysis of IgE reactivity to recombinant allergens from grass pollen and mite. Specific IgE was positive to Der p 2 (70%) and Der p 10 (55%) in African patients and Der p 2 (80%) and Der p 10 (10%) in European patients.22 The percentage of Der p 2 reactive sera in our study (73.3%) was not comparable because of population and climate differences among the three countries, each located in a different continent. Der p 10 sensitisation in our patients (15.8%) was closer to those Austrian subjects. This component is a tropomyosin, which is a protein that regulates muscle contraction and considered a pan-allergen highly conserved in invertebrates. Tropomyosins have over 80% amino acid sequence identity. Der p 10 cross-reacts with other invertebrate allergen sources like cockroach (Bla g 7), helminths (Ani s 3) and shrimp (Pen a 1).23,24 Fourteen subjects were positive to all these four allergen components (data not shown in the results). Possibly our patients presented different Der p 10 positivity rates due to different prevalence of helminthiasis, distinct eating habits and climate. Although Brazil is a developing country, subjects live in the urban area and in contrast with the African population, have higher socioeconomic condition, probably with a lower prevalence of helminths.
Seasonal allergic rhinoconjunctivitis has been recognised only in the last 40 years in Brazil. It is more common in adults than in children and is restricted to Southern regions of the country, contributing to the scarcity of studies on pollinosis.25 An epidemiological survey in a general population of adolescents and in adults by skin tests with Lolium multiflorum extract demonstrated sensitisation to this grass in 4.7% and 15.4%, respectively.26
Recently, 78 patients (aged 6–55 years) diagnosed with grass hay fever through positive SPT reactions to Lolium multiflorum underwent molecular allergy analysis.27 The frequency of recognition to Cyn d 1 (Cynodon dactylon) was 85% and to Phl p 1, 2, 4, 5, 6, 11, 12 (Phleum pratense) was 95%, 76%, 64%, 82%, 45%, 18%, 18%, respectively. The frequency of detection of IgE antibodies to those components in our study was much lower in children with perennial rhinitis: 16.8% to Cyn d 1 and 14.8%, 0.9%, 12.9%, 1.9%, 1.9%, 2.9%, 0.9% to Phl p 1, 2, 4, 5, 6, 11, and 12, respectively.
Pollen sensitisation rates vary in different regions according to the distribution of allergenic plants. Serum IgE antibodies to eight Phleum pratense molecules were tested in a birth cohort to investigate the evolution of IgE response to grass pollen in a German cohort. At age three years, IgE sensitisation predicted seasonal allergic rhinitis by age 12 years showing that sensitisation could start years before the symptoms. It is often initiated by Phl p 1 monosensitisation that becomes more complex with time in children affected by seasonal allergic rhinitis.28 The cross-sectional nature of our study performed on paediatric subjects distributed in a wide age range does not allow comparison with the German longitudinal cohort. It would be interesting to evaluate sensitisation to Phleum pratense over the years to verify the appearance of seasonal symptoms in our subjects.
Furthermore, weed and tree sensitisation rates were not relevant among our patients except for IgE antibodies in 9.9% to Pla a 2, a London Plane pollen allergen. All of them were equally positive to Pru p 3, a food allergen from peach whose protein is known as LTP (lipid transfer protein).29 Cross-reactivity between foods and pollens containing this LTP has been reported. Although our participants had been diagnosed with persistent rhinitis, seasonal exacerbations could not be ruled out, considering that they live in area where there are platanus and grass pollinosis. 25,30 LTPs are pan-allergens and markers of severe food allergic reactions.31 Subjects in this study may develop oral allergy symptoms (food allergy related to pollen) as they grow older.
In conclusion, our study showed the inhalant allergen determinants responsible for allergic sensitisation in a group of children and adolescents diagnosed with allergic respiratory diseases from Southern Brazil and reflected the local exposure to prevalent allergen sources (mites). Despite the high prevalence of asthma and rhinitis, our results in a small population sample reinforce the need for local studies throughout the world, as the sensitisation pattern varies according to the environment and selected population. Interpretation of results of molecular analysis must consider several factors, particularly the correlation with clinical symptoms and knowledge of IgE cross-reactivity between various allergen sources. Although SPT remains the cheapest and favoured method to detect IgE-mediated sensitivity to aeroallergens, the use of modern tools for sIgE detection such as microarray should be encouraged. That will enable to refine allergic diagnosis and impact on allergy prevention and specific immunotherapy.
Conflict of interestThe authors have no conflict of interest to declare.
FundingNone declared.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
The authors thank all the parents and children who participated in this study.