Rhinitis and rhinosinusitis are major concerns in public health. Mites are important aetiological agents in the tropics. The present study investigated the in vivo response to mite allergens in patients with rhinitis and rhinosinusitis.
MethodsAll patients with presumptive nasal allergy were included. Skin tests were done with inhalants and mite extracts. Patients were classified as allergic or non-allergic according to skin tests and history.
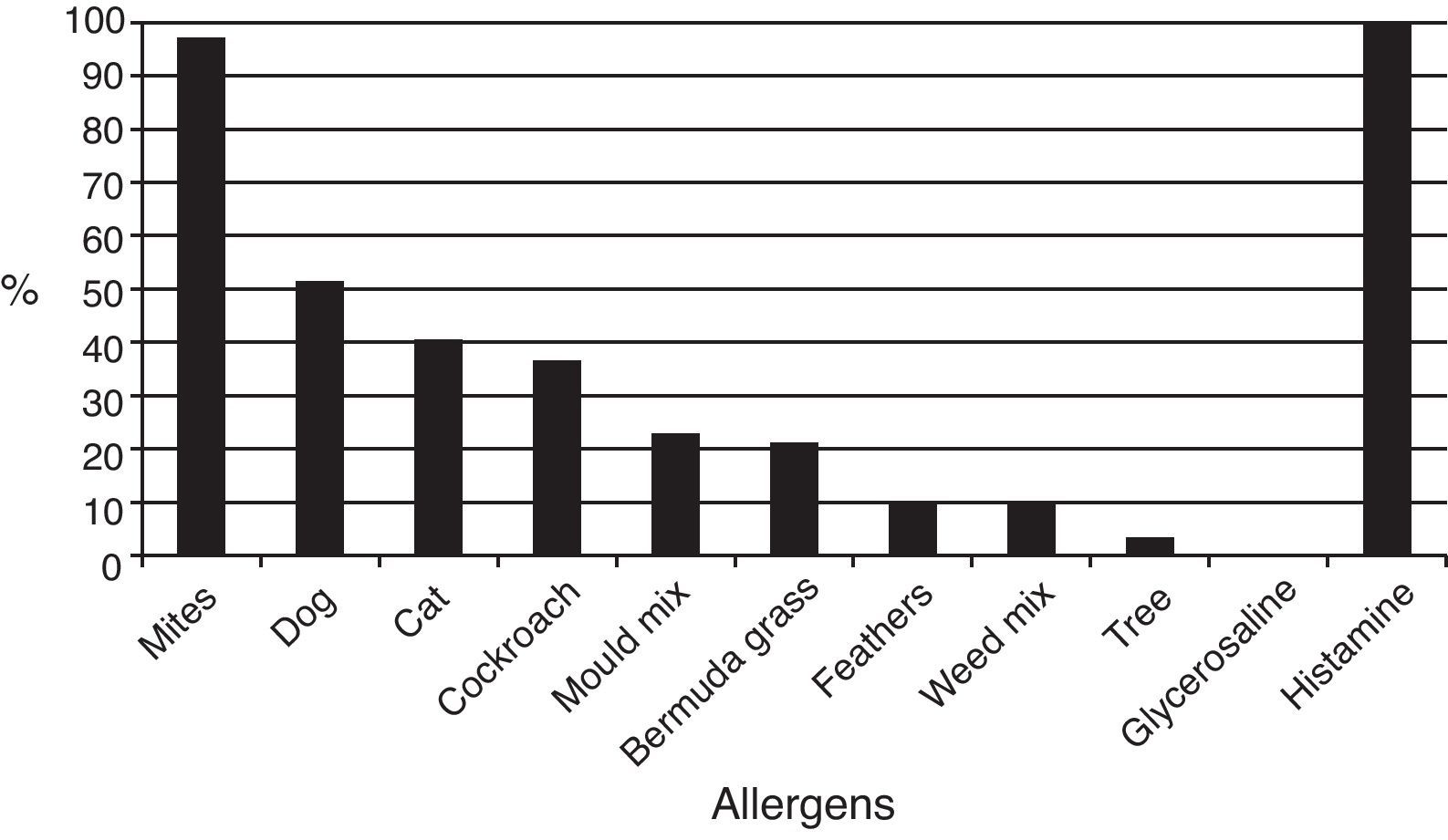
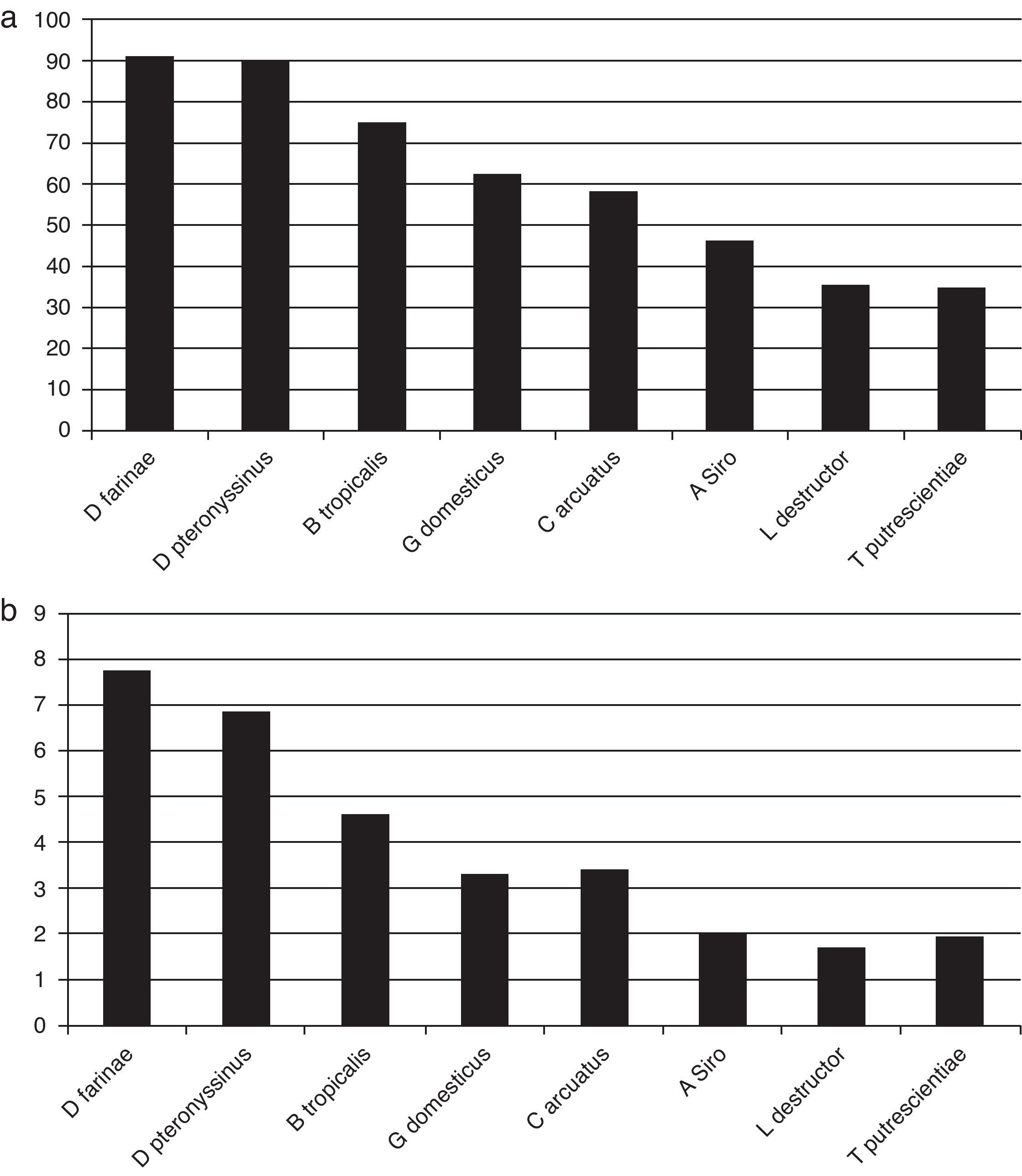
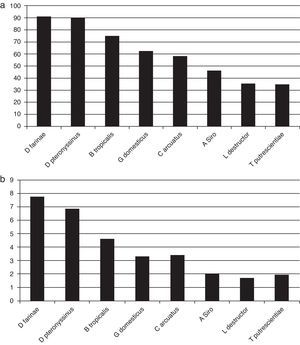
ResultsOut of 229 patients, 175 (76.4%) showed positive skin tests. Allergic patients showed positivity to mites in 97.1% of cases, 51.4% to dog dander; 40.5% to cat dander; 36.5% to German cockroach; 22.8% to moulds; and 21.1% to grass pollens. Dermatophagoides farinae induced responses in 90.8% of patients, D. pteronyssinus in 90.1%, Blomia tropicalis in 74.8%, Glycyphagus domesticus in 62.2%, Chortoglyphus arcuatus in 58.2%, Acarus siro in 46.2%, Lepidoglyphus destructor in 35.4%, and Tyrophagus putrescentiae in 35.0%. Higher correlations were found between skin test diameters induced by mites from the same family.
ConclusionsSensitisation to inhalant allergens is present in 76% of allergy clinics’ patients with rhinitis or rhinosinusitis. Our results confirm previous observations showing that mites constitute the most important cause of respiratory allergy in tropical settings and suggest that mite allergen cross-reactivity is responsible for the positivity of skin tests to mites not present in the patient's environment since the species Glycyphagus, Chortoglyphus, Acarus, Lepidoglyphus and Tyrophagus have not been found in Caracas house dust.
Allergic rhinitis is the most common form of non-infectious rhinitis, affecting between 10% and 30% of all adults and as many as 40% of children, with a tendency to continued prevalence increase.1 According to the ISAAC study, the prevalence of rhinitis and rhinoconjunctivitis in 13–14-year-old children has recently increased worldwide.2 Rhinosinusitis affects about 31 million subjects in the United States per year, and allergic and non-allergic rhinitis are the most common underlying causes.3 In Venezuela, the prevalence of current symptoms of rhinoconjunctivitis in 6–7-year-old children is 20.4% whereas in children aged 13–14 years it is 24.9%.4
In Latin America the prevalence of allergic rhinitis in children is high. ISAAC Phase Three Study reported 37.6% prevalence with a 0.8% yearly increase during the last few years.5 Under-reporting and decreased patient recognition of the disease have also been noticed.6 A high prevalence of sensitisation to mite allergens has been observed in patients with respiratory diseases living in various Latin American cities.7 In a previous study, we reported sensitisation rates of 97.2% for Dermatophagoides pteronyssinus and 91.6% for Blomia tropicalis in Venezuelan patients with rhinitis and/or asthma attending two allergy clinics in Caracas, Venezuela.8
The purpose of this study was to investigate the in vivo allergic response to eight mite allergenic extracts in patients with persistent rhinitis and rhinosinusitis living in a South American tropical city.
Materials and methodsPatientsThis was a prospective study where all patients with symptoms suggestive of rhinitis or rhinosinusitis referred to our allergy clinics between August 2010 and September 2011 were included after written informed consent was obtained. The protocol was approved by the Institutional Review Board of the participating institution. The clinical data on age, gender, previous diagnostic and surgical procedures, and comorbidities were obtained by direct patient questioning. The diagnosis of persistent rhinitis was done according to ARIA Guidelines,9 whereas the diagnosis of chronic rhinosinusitis was established according to the EAACI Position Paper on Rhinosinusitis and Nasal Polyps (EPOs).10 Patients were classified into allergic or non-allergic according to the presence or absence of at least one positive skin test to tested inhalant allergens.
Skin testsImmediate hypersensitivity skin tests were done by the prick method. Inhalant allergenic extracts were obtained from ALK Abelló (Madrid, Spain), while mite extracts were provided by Laboratorios Diater (Buenos Aires, Argentina). The following allergen extracts were tested: Dog dander (10HEP), Cat dander (10HEP, Feld 1 60μg/ml), Blatella germanica (1:100, w/v), Mould mix (1:20, w/v), Bermuda grass pollen (30HEP, containing Cyn d 1 30μg/ml), Weed pollen mix (30HEP), Feathers (1:100, w/v), Tree pollen mix (1:20, w/v), Dermatophagoides farinae (50,000HEP/ml) Dermatophagoides pteronyssinus (50.000HEP/ml), Blomia tropicalis (50.000HEP/ml), Glycyphagus domesticus (50.000PNU/ml), Chortoglyphus arcuatus (50.000PNU/ml), Acarus siro (50.000PNU/ml). Lepidoglyphus destructor (50.000PNU/ml), and Tyrophagus putrescentiae (50.000PNU/ml). Reading was performed after 20min of application and wheal sizes 3mm higher than negative control were considered as positive reactions. Glycerosaline solution and histamine phosphate 1mg/ml were employed as negative and positive controls, respectively.
Statistical analysisDifferences in percentages between the groups were compared by Fisher's exact test. Correlations were determined using the Pearson's test. All p values of less than 0.05 were considered statistically significant.
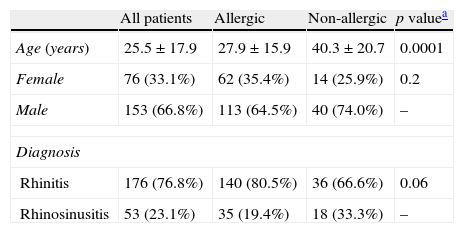
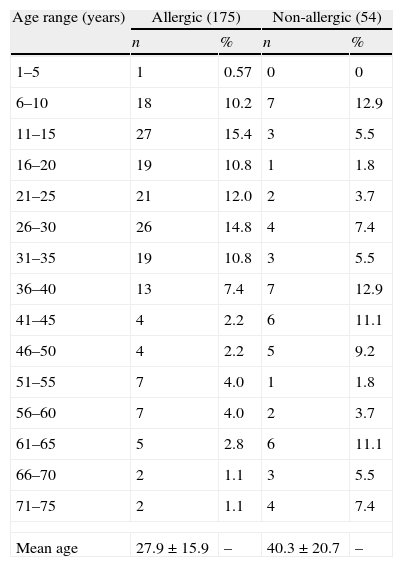
ResultsDemographic and clinical featuresTwo hundred and twenty-nine patients were included in this study, which included 153 male and 76 female patients, with mean age 25.5±17.9 years (range 5–76 years). One hundred and seventy-five (76.4%) were allergic, as demonstrated by means of skin test positivity to one or more inhalant allergens, and 54 (23.5%) were not allergic. Mean age was significantly higher in non-allergic patients (40.3±20.7 years versus 27.9±15.9 years, p=0.0001). No statistically significant differences were observed for gender (Table 1). Age distribution of allergic and non-allergic patients is presented in Table 2.
Demographic data and clinical diagnosis (n=229). Allergic 175 (76.4%), non-allergic 54 (23.5%).
| All patients | Allergic | Non-allergic | p valuea | |
| Age (years) | 25.5±17.9 | 27.9±15.9 | 40.3±20.7 | 0.0001 |
| Female | 76 (33.1%) | 62 (35.4%) | 14 (25.9%) | 0.2 |
| Male | 153 (66.8%) | 113 (64.5%) | 40 (74.0%) | – |
| Diagnosis | ||||
| Rhinitis | 176 (76.8%) | 140 (80.5%) | 36 (66.6%) | 0.06 |
| Rhinosinusitis | 53 (23.1%) | 35 (19.4%) | 18 (33.3%) | – |
Age distribution of allergic and non-allergic patients with rhinitis and rhinosinusitis.
| Age range (years) | Allergic (175) | Non-allergic (54) | ||
| n | % | n | % | |
| 1–5 | 1 | 0.57 | 0 | 0 |
| 6–10 | 18 | 10.2 | 7 | 12.9 |
| 11–15 | 27 | 15.4 | 3 | 5.5 |
| 16–20 | 19 | 10.8 | 1 | 1.8 |
| 21–25 | 21 | 12.0 | 2 | 3.7 |
| 26–30 | 26 | 14.8 | 4 | 7.4 |
| 31–35 | 19 | 10.8 | 3 | 5.5 |
| 36–40 | 13 | 7.4 | 7 | 12.9 |
| 41–45 | 4 | 2.2 | 6 | 11.1 |
| 46–50 | 4 | 2.2 | 5 | 9.2 |
| 51–55 | 7 | 4.0 | 1 | 1.8 |
| 56–60 | 7 | 4.0 | 2 | 3.7 |
| 61–65 | 5 | 2.8 | 6 | 11.1 |
| 66–70 | 2 | 1.1 | 3 | 5.5 |
| 71–75 | 2 | 1.1 | 4 | 7.4 |
| Mean age | 27.9±15.9 | – | 40.3±20.7 | – |
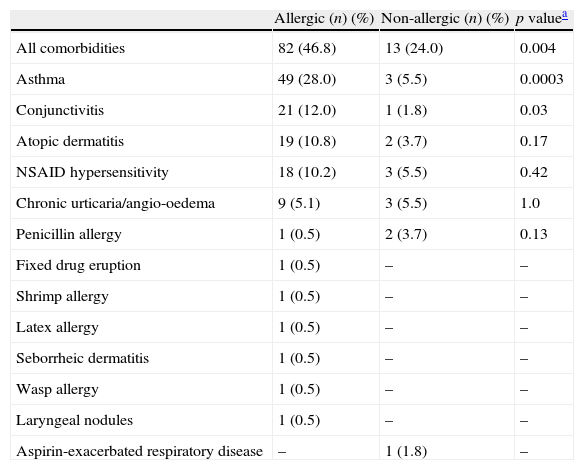
Rhinosinusitis was more frequent in non-allergic patients, and rhinitis was more prevalent in allergic subjects, although these differences were not statistically significant (Table 1). The comorbidities observed in allergic and non-allergic patients are shown in Table 3. Eighty-two allergic patients (46.8%) and 13 non-allergic patients (24.0%) had comorbidities (p=0.004), with asthma, conjunctivitis, atopic dermatitis, NSAID hypersensitivity, and chronic spontaneous urticaria/angio-oedema being the most frequent.
Comorbidities.
| Allergic (n) (%) | Non-allergic (n) (%) | p valuea | |
| All comorbidities | 82 (46.8) | 13 (24.0) | 0.004 |
| Asthma | 49 (28.0) | 3 (5.5) | 0.0003 |
| Conjunctivitis | 21 (12.0) | 1 (1.8) | 0.03 |
| Atopic dermatitis | 19 (10.8) | 2 (3.7) | 0.17 |
| NSAID hypersensitivity | 18 (10.2) | 3 (5.5) | 0.42 |
| Chronic urticaria/angio-oedema | 9 (5.1) | 3 (5.5) | 1.0 |
| Penicillin allergy | 1 (0.5) | 2 (3.7) | 0.13 |
| Fixed drug eruption | 1 (0.5) | – | – |
| Shrimp allergy | 1 (0.5) | – | – |
| Latex allergy | 1 (0.5) | – | – |
| Seborrheic dermatitis | 1 (0.5) | – | – |
| Wasp allergy | 1 (0.5) | – | – |
| Laryngeal nodules | 1 (0.5) | – | – |
| Aspirin-exacerbated respiratory disease | – | 1 (1.8) | – |
CT scans of paranasal sinuses were performed in 29 allergic and 14 non-allergic patients. Sinusitis, septum deviation and polyposis were the most common abnormalities present in both groups of patients. Previous history of surgical treatment was present in 34 allergic (45 procedures) and 12 non-allergic patients (28 procedures), including functional endoscopic nasal surgery, adenoidectomy, tonsillectomy and orthodontic surgery.
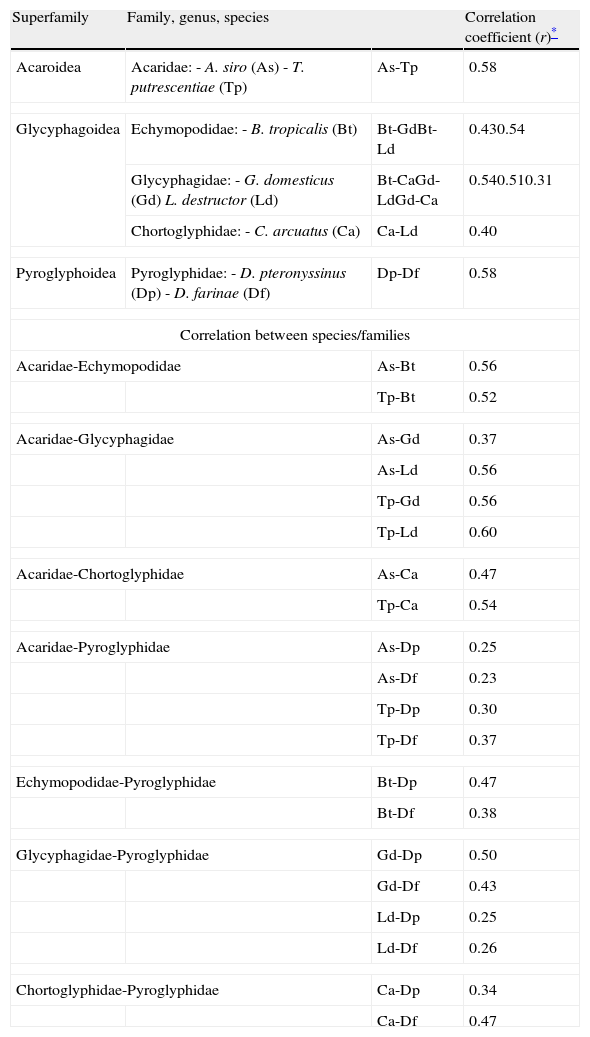
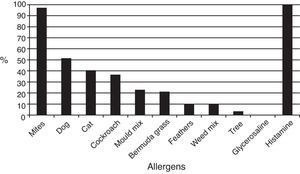
Skin testsThe results of prick tests to inhalant allergens are shown in Fig. 1. A large proportion of allergic patients (97.1%) responded to one or more mite extracts. About half of them showed responses to dog dander extract, followed by cat dander, Blatella germanica, mould mix, and Bermuda grass pollen. Responses to feathers, weed pollen, and tree pollen were observed less often. Fig. 2 presents the results of skin tests to extracts from eight mite species. It can be observed that responses to D. farinae and pteronyssinus, B. tropicalis, G. domesticus and C. arcuatus were more frequently present, whereas a lower number of patients responded to A. siro, L. destructor, and T. putrescentiae. Monosensitisations to only one mite species were as follows: three patients to B. tropicalis, three to D. farinae, two to D. pteronyssinus, and two to A. siro. The correlation between the wheal diameters induced by the different mite allergenic extracts was studied by Pearson's method (Table 4). Although all correlations were statistically significant, intra-family correlations showed a tendency to higher coefficients (r≥0.5) than inter-family and interspecies correlations.
Correlations between wheal diameters induced by mite allergenic extracts in patients with allergic rhinitis/rhinosinusitis.
| Superfamily | Family, genus, species | Correlation coefficient (r)* | |
| Acaroidea | Acaridae:- A. siro (As)- T. putrescentiae (Tp) | As-Tp | 0.58 |
| Glycyphagoidea | Echymopodidae:- B. tropicalis (Bt) | Bt-GdBt-Ld | 0.430.54 |
| Glycyphagidae:- G. domesticus (Gd)L. destructor (Ld) | Bt-CaGd-LdGd-Ca | 0.540.510.31 | |
| Chortoglyphidae:- C. arcuatus (Ca) | Ca-Ld | 0.40 | |
| Pyroglyphoidea | Pyroglyphidae:- D. pteronyssinus (Dp)- D. farinae (Df) | Dp-Df | 0.58 |
| Correlation between species/families | |||
| Acaridae-Echymopodidae | As-Bt | 0.56 | |
| Tp-Bt | 0.52 | ||
| Acaridae-Glycyphagidae | As-Gd | 0.37 | |
| As-Ld | 0.56 | ||
| Tp-Gd | 0.56 | ||
| Tp-Ld | 0.60 | ||
| Acaridae-Chortoglyphidae | As-Ca | 0.47 | |
| Tp-Ca | 0.54 | ||
| Acaridae-Pyroglyphidae | As-Dp | 0.25 | |
| As-Df | 0.23 | ||
| Tp-Dp | 0.30 | ||
| Tp-Df | 0.37 | ||
| Echymopodidae-Pyroglyphidae | Bt-Dp | 0.47 | |
| Bt-Df | 0.38 | ||
| Glycyphagidae-Pyroglyphidae | Gd-Dp | 0.50 | |
| Gd-Df | 0.43 | ||
| Ld-Dp | 0.25 | ||
| Ld-Df | 0.26 | ||
| Chortoglyphidae-Pyroglyphidae | Ca-Dp | 0.34 | |
| Ca-Df | 0.47 | ||
General practitioners and other physicians tend to ascribe a diagnosis of allergic rhinitis to patients who present chronic or recurrent nasal symptoms, mainly rhinorrhoea, nasal congestion and itching, or sneezing. It was then interesting to quantify the proportion of patients referred to the allergist with a presumptive diagnosis of allergic rhinitis in which a demonstrable allergic sensitisation to aeroallergens is present. Although about three quarters of the patients in this study were allergic, it is also important to notice that about one quarter of them did not react to the most common environmental allergens and were classified as non-allergic or idiopathic.
Patients with non-allergic rhinitis or rhinosinusitis are generally thought to suffer a more severe condition. However, in the present investigation, comorbidities (including asthma and conjunctivitis) were more frequently present in the allergic group. This observation emphasizes the relevance of comorbidities in determining the quality of life of patients suffering from allergic rhinitis and rhinosinusitis, as has been indicated in recent documents.1,9
The results of skin tests confirmed previous studies from various Latin American cities, including Caracas, where mites are the most important allergenic source for the induction of respiratory allergy,6,11,12 followed by allergens from pets, moulds, and cockroaches. Pollens from grasses, weeds, and trees are sensitising agents in a lower proportion of patients. This is more remarkable in tropical/subtropical locations with higher temperature and relative humidity, such as Venezuela. In a recent study, Caplin et al. observed that the species diversity and absolute mite counts present in mattress dusts were significantly higher in Caracas than in Corpus Christi, Texas, despite both cities being located in the Caribbean region close to the seashore.13
All mite species present in the home environment and capable of inducing IgE-mediated sensitisation are called “domestic mites”. However, the name “storage mites” is still used to designate mite species found in house dust that do not belong to the family Pyroglyphidae. Previous studies concerning the acarofauna of Caracas are scarce. Hurtado and Parini reported the following species in bedding dust: D. pteronyssinus, D. farinae, E. maynei, B. tropicalis, and Cheyletus spp.14 Capriles-Hulett et al. observed, in addition to the above mentioned species, the following: Suidasia spp., Anoetidae, Tarsonemus, Demodex, Prostigmata and Oribatidae.15 We have also found D. pteronyssinus, Aleuroglyphus ovatus, and Suidasia spp. contaminating wheat flour and inducing oral mite anaphylaxis in Venezuelan patients.16
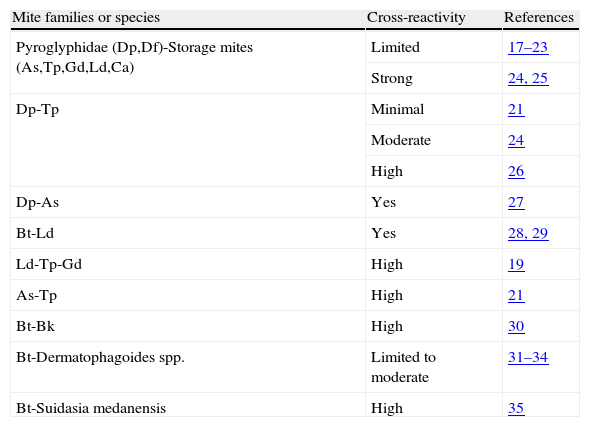
Since allergic patients with rhinitis or rhinosinusitis recruited into the present study reacted to mite species not previously observed in Caracas’ homes, such as G. domesticus, C. arcuatus, A. siro, L. destructor and T. putrescentiae, the most likely explanation for such reactions would be the cross-reactivity which is a common feature among mite allergens, especially in those from taxonomically related species. In Table 5 a summary of cross-reactions described for domestic mites is shown. Many allergens, especially those from group 2, have been described in the majority of mite species included in this investigation.36
Cross-reactions between domestic mites.
| Mite families or species | Cross-reactivity | References |
| Pyroglyphidae (Dp,Df)-Storage mites (As,Tp,Gd,Ld,Ca) | Limited | 17–23 |
| Strong | 24, 25 | |
| Dp-Tp | Minimal | 21 |
| Moderate | 24 | |
| High | 26 | |
| Dp-As | Yes | 27 |
| Bt-Ld | Yes | 28, 29 |
| Ld-Tp-Gd | High | 19 |
| As-Tp | High | 21 |
| Bt-Bk | High | 30 |
| Bt-Dermatophagoides spp. | Limited to moderate | 31–34 |
| Bt-Suidasia medanensis | High | 35 |
The intrafamily correlation of skin test results suggests strong antigenic cross-reactions especially in the cases of A. siro and T. putrescentiae (r=0.58), B. tropicalis and L. destructor (r=0.54), B. tropicalis and C. arcuatus (r=0.54), G. domesticus and L. destructor (r=0.51), and D. pteronyssinus and D. farinae (r=0.58) (Table 4). The correlations between A. siro and B. tropicalis (r=0.56), T. putrescentiae and B. tropicalis (r=0.52), A. siro and L. destructor (r=0.56), T. putrescentiae and G. domesticus (r=0.60), T. putrescentiae and C. arcuatus (r=0.54), and G. domesticus and D. pteronyssinus (r=0.50) are more difficult to explain, since those combinations include mites from different families. It is, however, important to notice that strong inter-family cross-reactions such as between T. putrescentiae and L. destructor have been observed (Table 5).19
Another observation supporting cross-reactions as the cause of skin test responses to mites not previously found in Caracas (Glycyphagus domesticus, Chortoglyphus arcuatus, Acarus siro, Lepidoglyphus destructor and Tyrophagus putrescentiae) is that monosensitisation to only one mite species was present in very few patients in the present investigation.
Recently, cross-reactions between mite allergens and helminth constituents have been described, especially related to tropomyosin and glutathione-s-transferases. These cross-reactions may be important confounding factors for the diagnosis of allergic sensitisation in the tropics, and therefore our results should be interpreted cautiously.37 Also, our present observations should be considered with some caution because non-standardised extracts of different potency were used for skin testing.
In conclusion, sensitisation to inhalant allergens is present in 76.4% of patients referred to allergy clinics with persistent rhinitis or chronic rhinosinusitis. Mites are the major allergen source in this tropical environment. Comorbidities were more frequently present in allergic than in non-allergic patients. Although a large proportion of patients reacted to storage mite extracts, those reactions are likely related to shared allergenic components inducing cross-reactions more than to exposure to those species.
Ethical disclosuresRight to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Financial supportInvestigator's funds.
Conflicts of interestLaboratorios Diater (Buenos Aires, Argentina) has donated the mite allergenic extracts used in this study.
Authors are grateful to Laboratorios Diater (Buenos Aires, Argentina) for the donation of the mite allergenic extracts used in this study.