Basophil activation test (BAT) and immunoassays are the most widely used in vitro tests to diagnose IgE-mediated allergic reactions to penicillin. However, studies to determine if one test is interdependent from another are limited.
ObjectiveThe present study aimed to measure the agreement between BAT and immunoassay in diagnosis of penicillin allergy.
MethodBAT was performed using penicillin G (Pen G), penicillin V (Pen V), penicilloyl-polylysine (PPL), minor determinant mix (MDM), amoxicillin (Amx) and ampicillin (Amp) in 25 patients. Immunoassay of total IgE (tIgE) and specific IgE (sIgE) antibodies to Pen G, Pen V, Amx and Amp were quantified. Skin prick test (SPT) using PPL-MDM, Amx, Amp and Clavulanic acid were also performed.
ResultsMinimal agreement was observed between BAT and immunoassay (k=0.25). Of two BAT-positive patients, one patient is positive to Amx (59.27%, SI=59) and Amp (82.32%, SI=82) but sIgE-negative to all drug tested. This patient is also SPT-positive to both drugs. Another patient is BAT-positive to Pen G (10.18%, SI=40), Pen V (25.07%, SI=100) and Amp (19.52%, SI=79). In sIgE immunoassay, four patients were sIgE-positive to at least one of the drugs tested. The sIgE level of three patients was between low and moderate and they were BAT-negative. One BAT-positive patient had a high level of sIgE antibodies (3.50–17.5kU/L) along with relatively high specific to total IgE ratio ≥0.002 (0.004–0.007).
ConclusionsThe agreement between BAT and immunoassay is minimal. Performing both tests provides little increase in the sensitivity of allergy diagnosis work-up for immediate reactions to penicillin.
Mislabeling of penicillin allergy is an increasingly challenging clinical issue which may lead to increased antibiotic resistance and healthcare costs. Most patients labeled as penicillin allergy are not really allergic to penicillin.1 It was estimated that only 15.8–28.6% of patients labeled as penicillin allergy are genuinely allergic.2,3 Individuals with a history of allergy to penicillin, particularly self-reported patients, should be evaluated and subjected to routine penicillin allergy testing to ideally prevent mislabeling.4 Such individuals may be evaluated by different methods that should include the clinical history, skin tests, in vitro quantification of specific IgE-antibodies, and drug provocation tests.5
With the advancement of flow cytometry technique, basophil activation test (BAT) has been proposed as a useful in vitro assay for the diagnosis of penicillin allergy. The BAT is a functional assay that measures IgE function which is the ability to induce activation of basophils in the presence of allergen. Basophil activation is usually assessed by determining CD63 or CD203c expression on the surface of basophils.6
Determination of total and specific IgE (sIgE) antibodies by immunoassay is another alternative in vitro method to diagnose allergic reactions to penicillin.7 This method however, measures both functional IgE (capable of activating mast cells and basophils by binding to the Fc region of IgE I (FcɛRI) on the cell surface) and non-functional IgE.8 Nevertheless, the serum level of sIgE antibodies may decline rapidly, in which the test often becomes negative within 6 months to 3 years after the last exposure.9
The present study aims to compare the diagnostic performance of BAT and immunoassay in the diagnosis of penicillin allergy by measuring the agreement between the tests.
Materials and methodsSubjectsTwenty-five adult patients referred to Allergy Clinic, Hospital Kuala Lumpur with suggestive clinical history of immediate allergic reactions to penicillin antibiotics within 1–12 months of reactions (median=3 months) were recruited. Patients were evaluated for penicillin allergy using the European Network for Drug Allergy (ENDA) recommended clinical questionnaire.10 Patients who received anti-IgE and antihistamines within 24h of consultation were not included. Twenty-five volunteered individuals without any history of drug allergy were included as tolerant controls. This study was approved by the Medical Research of Ethics Committee of the Ministry of Health in Malaysia (KKM/NIHSEC/P13-901, NMRR-13-922-17589) and informed consent for all testing was obtained from all patients and controls.
Skin Prick Test (SPT)SPT was performed on 24 patients according to conventional techniques using freshly prepared mixtures of benzylpenicilloyl octa-l-lysine (PPL) and sodium benzylpenilloate (MDM), sodium amoxicillin (Amx), potassium clavulanate (Clav A) and an in-house preparation of ampicillin (Amp). Histamine hydrochloride (10mg/ml) was used as the positive control and 0.9% saline solution as the negative control. All commercial allergens and controls were purchased from Diater LABORATORIOS S.A, Madrid, Spain. SPT is considered positive when the wheal diameter equals 3mm or greater than the negative control within 20min.
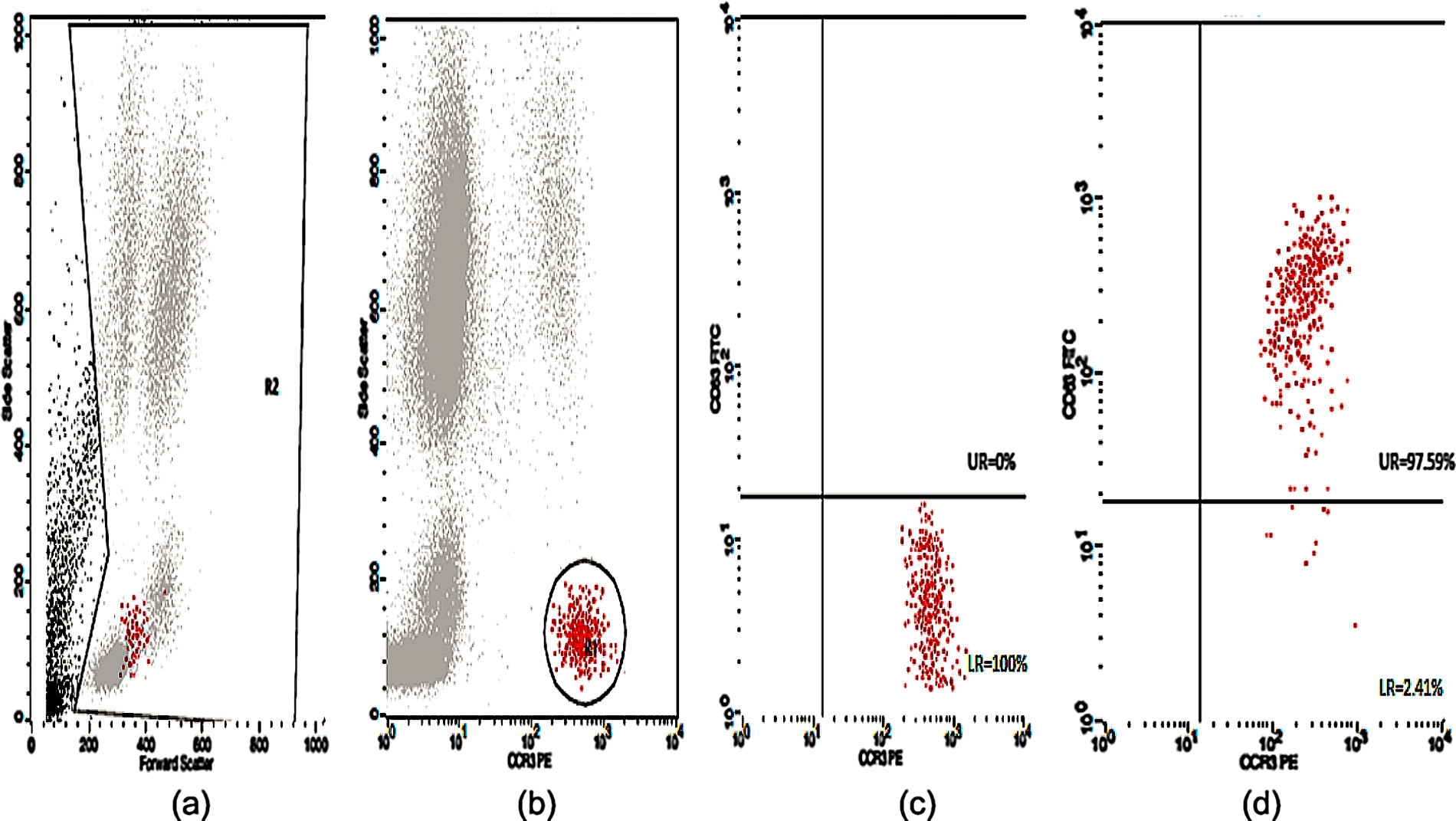
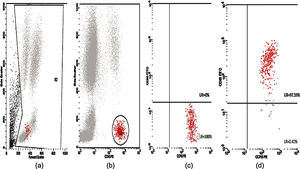
Basophil Activation Test (BAT)Peripheral blood samples in ethylene-diamine tetra acetic acid (EDTA) tubes were tested for CD63 expression. The flow cytometric analysis of the in vitro activated basophils was carried out using Flow CAST® (Bühlmann Laboratories AG, Schönenbuch, Switzerland) following the manufacturer's instructions and cut-off recommendation. Briefly, basophil stimulation in whole blood were performed immediately (within 2h) upon blood collection using drug allergens by CAST® (Bühlmann Laboratories AG, Schönenbuch, Switzerland) namely benzylpenicilloyl-polylysine (PPL), minor-determinant mix (MDM), penicillin G (Pen G), penicillin V (Pen V), ampicillin (Amp) and amoxicillin (Amx). Stimulation buffer and mixture of anti-FcɛRI and fMLP were used as negative and positive controls, respectively. A mixture of anti-CCR3-PE and anti-CD63-FITC was used as staining reagent. The samples were analyzed using CellQuest® software (FACSCalibur BD Analyser) within 2h. The number of events acquired was set to contain at least 400 basophils. Percentage of activated basophils (CD63 positive cells) were expressed for each drug. The stimulation index (SI) was defined as the activated basophil percentage after drug stimulation/basally active basophil percentage. A SI ≥2 and an absolute activated basophil percentage ≥5 were considered positive.
Total and sIgE immunoassayQuantification of total IgE in 18 patients and specific IgE antibodies to c1 (penicilloyl G), c2 (penicilloyl V), c5 (ampicilloyl) and c6 (amoxilloyl) in all patients’ plasma were performed by fluoroenzyme immunoassay (FEIA) method using UniCAP® Phadia 250 systems (Thermo Fischer Scientific, Uppsala, Sweden) following the manufacturer's instructions. Specific IgE results were obtained by direct comparison with standards run in parallel, considering a value of sIgE ≥0.35kU/L as positive. The ratio of specific to total IgE was also determined in patients with serum total IgE >200kU/l.
Statistical analysisAgreement between BAT and immunoassay was calculated using Cohen's kappa Index (k) and interpreted using the interpretation described by McHugh (2012).11 Cohen's kappa Index was calculated as k=(pa−pe)/(1−pe), where: pa=proportions of observation in agreement and pe=proportions of agreement due to chance.
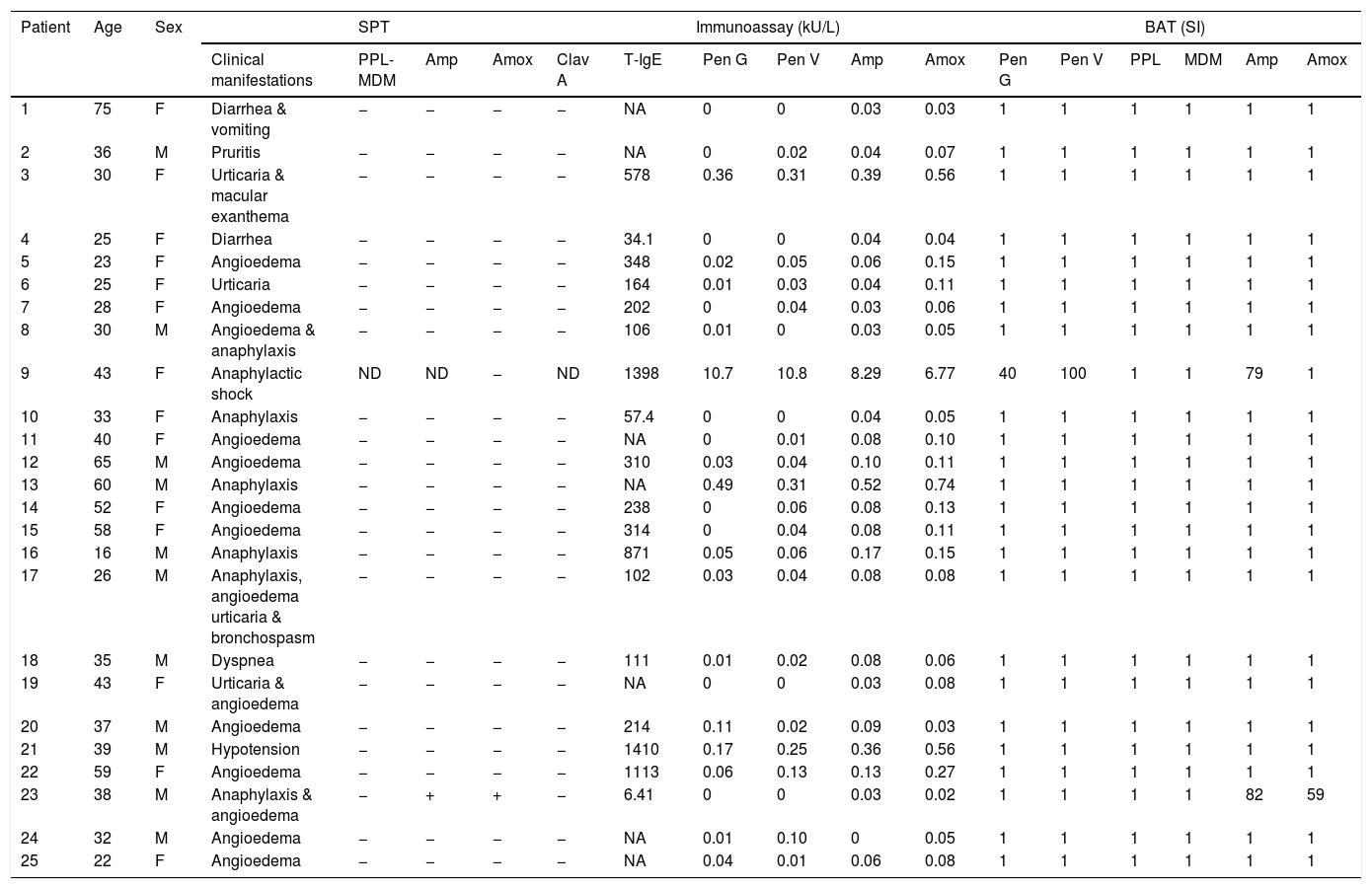
ResultsPatient's clinical manifestations and SPTOf 25 patients, 14 were females and 11 were males, with a median age of 36 years old (interquartile range (IR): 28–43). Patients’ clinical manifestations, SPT, immunoassays and BAT results are shown in Table 1. Clinical manifestations included angioedema in 14 cases (56%), anaphylaxis in seven (28%), urticaria in four (16%), diarrhea in two (8%), followed by vomiting, macular exanthema, pruritis, bronchospasm, dyspnea and hypotension in each case (4%). Only one patient (patient 23) was SPT-positive to Amx and Amp.
Patient's clinical manifestations with SPT, immunoassay and BAT results.
| Patient | Age | Sex | SPT | Immunoassay (kU/L) | BAT (SI) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical manifestations | PPL-MDM | Amp | Amox | Clav A | T-IgE | Pen G | Pen V | Amp | Amox | Pen G | Pen V | PPL | MDM | Amp | Amox | |||
| 1 | 75 | F | Diarrhea & vomiting | − | − | − | − | NA | 0 | 0 | 0.03 | 0.03 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 36 | M | Pruritis | − | − | − | − | NA | 0 | 0.02 | 0.04 | 0.07 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3 | 30 | F | Urticaria & macular exanthema | − | − | − | − | 578 | 0.36 | 0.31 | 0.39 | 0.56 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | 25 | F | Diarrhea | − | − | − | − | 34.1 | 0 | 0 | 0.04 | 0.04 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5 | 23 | F | Angioedema | − | − | − | − | 348 | 0.02 | 0.05 | 0.06 | 0.15 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6 | 25 | F | Urticaria | − | − | − | − | 164 | 0.01 | 0.03 | 0.04 | 0.11 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7 | 28 | F | Angioedema | − | − | − | − | 202 | 0 | 0.04 | 0.03 | 0.06 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8 | 30 | M | Angioedema & anaphylaxis | − | − | − | − | 106 | 0.01 | 0 | 0.03 | 0.05 | 1 | 1 | 1 | 1 | 1 | 1 |
| 9 | 43 | F | Anaphylactic shock | ND | ND | − | ND | 1398 | 10.7 | 10.8 | 8.29 | 6.77 | 40 | 100 | 1 | 1 | 79 | 1 |
| 10 | 33 | F | Anaphylaxis | − | − | − | − | 57.4 | 0 | 0 | 0.04 | 0.05 | 1 | 1 | 1 | 1 | 1 | 1 |
| 11 | 40 | F | Angioedema | − | − | − | − | NA | 0 | 0.01 | 0.08 | 0.10 | 1 | 1 | 1 | 1 | 1 | 1 |
| 12 | 65 | M | Angioedema | − | − | − | − | 310 | 0.03 | 0.04 | 0.10 | 0.11 | 1 | 1 | 1 | 1 | 1 | 1 |
| 13 | 60 | M | Anaphylaxis | − | − | − | − | NA | 0.49 | 0.31 | 0.52 | 0.74 | 1 | 1 | 1 | 1 | 1 | 1 |
| 14 | 52 | F | Angioedema | − | − | − | − | 238 | 0 | 0.06 | 0.08 | 0.13 | 1 | 1 | 1 | 1 | 1 | 1 |
| 15 | 58 | F | Angioedema | − | − | − | − | 314 | 0 | 0.04 | 0.08 | 0.11 | 1 | 1 | 1 | 1 | 1 | 1 |
| 16 | 16 | M | Anaphylaxis | − | − | − | − | 871 | 0.05 | 0.06 | 0.17 | 0.15 | 1 | 1 | 1 | 1 | 1 | 1 |
| 17 | 26 | M | Anaphylaxis, angioedema urticaria & bronchospasm | − | − | − | − | 102 | 0.03 | 0.04 | 0.08 | 0.08 | 1 | 1 | 1 | 1 | 1 | 1 |
| 18 | 35 | M | Dyspnea | − | − | − | − | 111 | 0.01 | 0.02 | 0.08 | 0.06 | 1 | 1 | 1 | 1 | 1 | 1 |
| 19 | 43 | F | Urticaria & angioedema | − | − | − | − | NA | 0 | 0 | 0.03 | 0.08 | 1 | 1 | 1 | 1 | 1 | 1 |
| 20 | 37 | M | Angioedema | − | − | − | − | 214 | 0.11 | 0.02 | 0.09 | 0.03 | 1 | 1 | 1 | 1 | 1 | 1 |
| 21 | 39 | M | Hypotension | − | − | − | − | 1410 | 0.17 | 0.25 | 0.36 | 0.56 | 1 | 1 | 1 | 1 | 1 | 1 |
| 22 | 59 | F | Angioedema | − | − | − | − | 1113 | 0.06 | 0.13 | 0.13 | 0.27 | 1 | 1 | 1 | 1 | 1 | 1 |
| 23 | 38 | M | Anaphylaxis & angioedema | − | + | + | − | 6.41 | 0 | 0 | 0.03 | 0.02 | 1 | 1 | 1 | 1 | 82 | 59 |
| 24 | 32 | M | Angioedema | − | − | − | − | NA | 0.01 | 0.10 | 0 | 0.05 | 1 | 1 | 1 | 1 | 1 | 1 |
| 25 | 22 | F | Angioedema | − | − | − | − | NA | 0.04 | 0.01 | 0.06 | 0.08 | 1 | 1 | 1 | 1 | 1 | 1 |
SPT: Skin Prick Test; BAT: Basophil Activation Test; T-IgE: Total-IgE; ND: not done; Pen G: Penicillin G; Pen V: Penicillin V; Amp: Ampicillin; Amox: Amoxicillin; Clav A: Clavulanic acid; PPL: benzylpenicilloyl-polylysine; MDM: minor determinant mix; ‘+’: positive result; ‘−’: negative result.
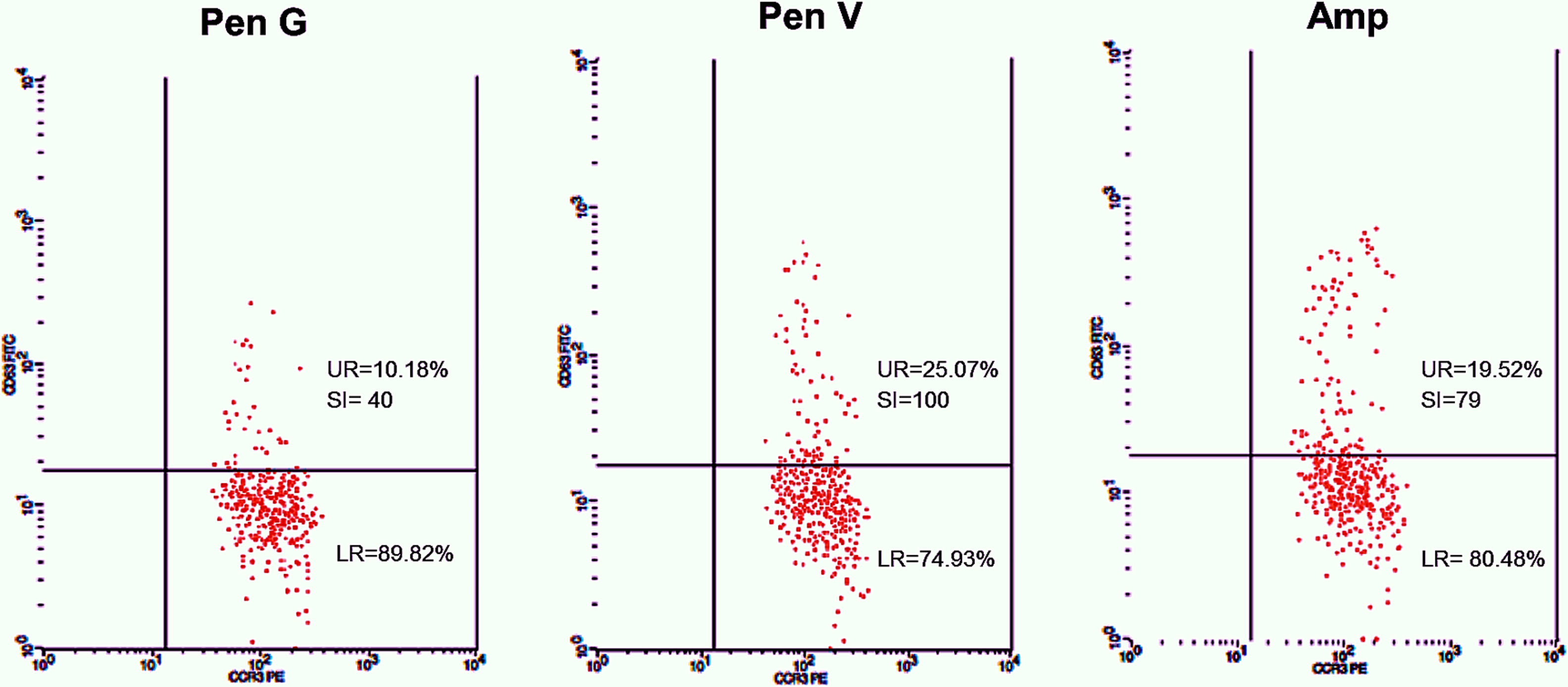
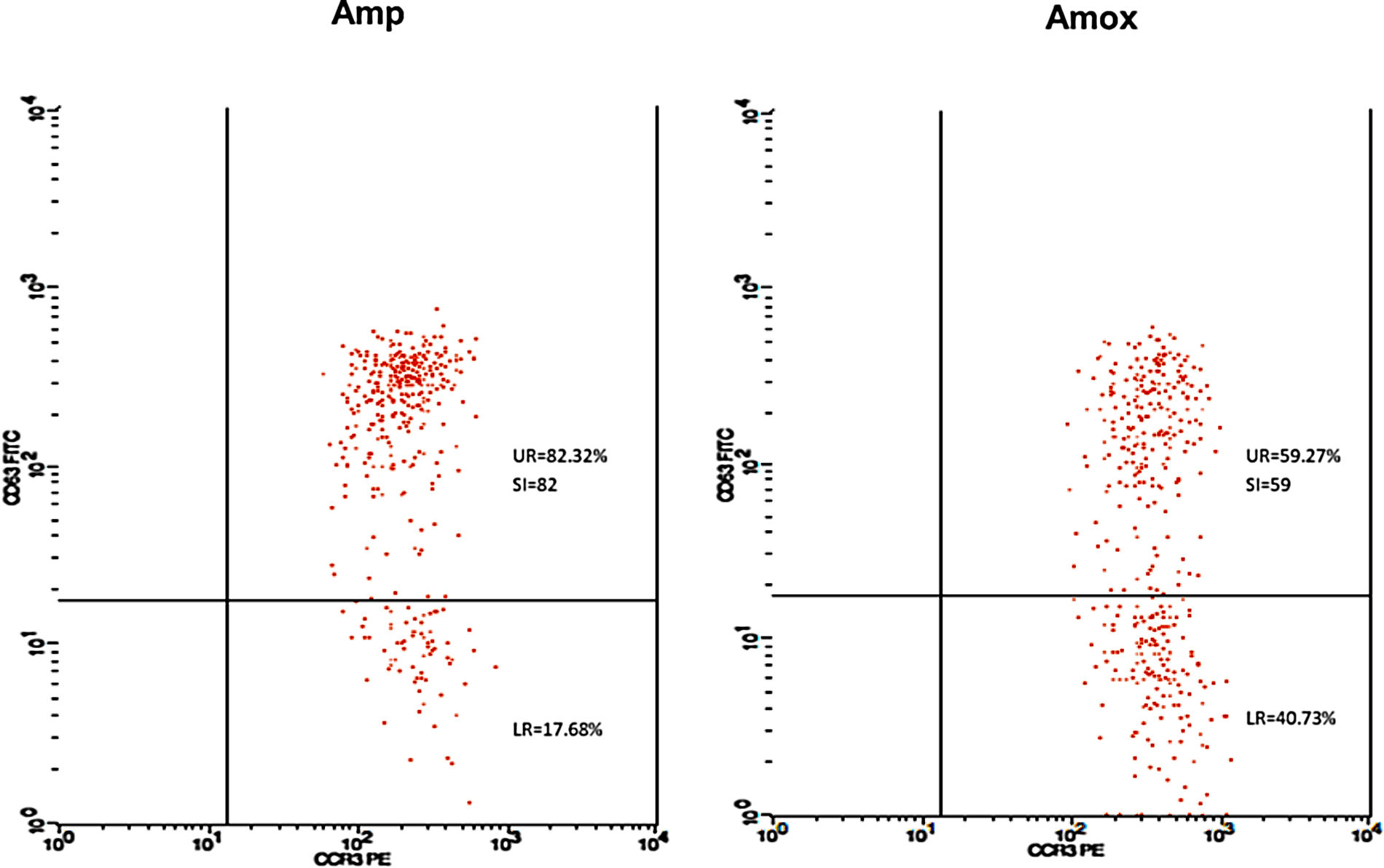
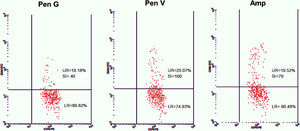
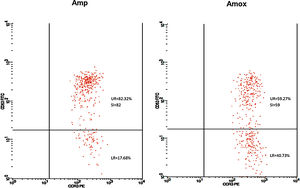
Twenty-five cases with equally numbered tolerant controls were evaluated for BAT. The basophil gating strategy is shown in Fig. 1. Two patients (patients 9 and 23) positively expressed CD63-FITC as shown in Figs. 2 and 3, respectively. Patient 9 positively expressed CD63 on its activated basophil when tested with Pen G (10.18%, SI=40), Pen V (25.07%, SI=100) and Amp (19.52%, SI=79). The results were in concordance with immunoassay results (Pen G=10.7kU/L, Pen V=10.8kU/L, Amp=8.29kU/L) except for Amx (6.77kU/L). Patient 23 positively expressed CD63 on activated basophil tested with Amp (82.32%, SI=82) and Amx (59.27%, SI=59). Interestingly, patient 23 was sIgE-negative (sIgE ≤0.35kU/l) to the entire drug tested. Minimal agreement between BAT and immunoassay results was observed (k=0.25).
Total IgE was elevated (≥100kU/L) in 15 (83%) of 18 patients. Four patients (patients 3, 9, 13 and 21) were sIgE positive (≥0.35kU/L) to at least one of the drugs tested. Of the four patients, three patients (patients 3, 13 and 21) had an sIgE level between low (sIgE level: 0.35–0.69kU/L) and moderate (sIgE level: 0.70–3.49kU/L). Only one patient (patient 9) with high sIgE antibody level (sIgE level: 3.50–17.5kU/L) showed a positive BAT. This patient also had relatively high specific to total IgE ratio ≥0.002 (0.004–0.007).
DiscussionA reliable in vitro test is very important to diagnose true penicillin allergy in order to prevent mislabeling. Currently, a provocation test with the culprit drug is still considered as the ‘gold standard’ in confirming drug hypersensitivity reactions. However, this has proven problematic in cases with life-threatening reactions and contraindicated in patients with severe cutaneous adverse reactions. As such, immunoassays and BAT have been recommended in some European countries to overcome the problems.12 However, studies to determine if one test is complementary or independent from another are rather limited.
In the present study, the agreement between immunoassay and BAT is relatively low (k=0.25). Only one of two BAT-positive patients exhibited a correlation with the immunoassay. False negative and false positive results might explain the discrepancies between results as both tests have been shown to give false results due to relatively low sensitivity. The average sensitivity and specificity of BAT are 51.7% and 89.2% respectively, while for immunoassay, 50.1% and 81.01%, respectively.13 A recent study has reported high BAT specificity (90%) with relatively low sensitivity (33.6%) suggesting a high variance of BAT sensitivities between studies.14 That same study examining 37 penicillin allergic patients demonstrated moderate concordance (k=0.4) between BAT and immunoassay; four patients had both BAT and immunoassay positive and two patients had positive BAT but negative immunoassay. However, a lower sIgE cut-off was utilized in their study compared to the present study, hence the difference of agreement value.
Our study also showed more patients being detected using immunoassay compared to BAT. It is known that sIgE patients suspected of penicillin allergy with a positive immunoassay result can be directed to a cross-reactive epitope, phenylethylamine, an allergenic structure related to penicillin but different from major and minor allergenic epitope. These false positive tests may limit the value of the immunoassay for diagnosis of penicillin allergy. On the other hand, BAT sensitivity is also low, this may be attributed to the inability of penicillin-derived monovalent allergenic determinants in activating basophils due to inhibition of the IgE cross-linking.15
When considering the severity of clinical symptoms, the present study demonstrated eight patients with severe clinical reactions (anaphylaxis/anaphylactic shock/hypotension). Both BAT-positive patients suffered from anaphylactic symptoms although only one patient showed consistent immunoassay results. We found that immunoassay appeared to be particularly useful for patients with severe reactions, as 75% of the immunoassay-positive patients suffered severe reactions. Indeed, obtaining a detailed clinical history is an essential step in complementing immunoassay and BAT. It facilitates the decision whether to perform a drug provocation test in high-risk patients, which will ultimately increase diagnosis accuracy.
Fair agreement (k=0.35) between BAT and skin tests was also observed in another study demonstrating the ability of BAT to detect five patients with a positive history but negative skin tests.16 In our study, one BAT-positive patient was SPT-positive in both drugs (amoxicillin and ampicillin). Meanwhile, SPT to another patient could not be performed due to an issue with consent. Although only one patient was detected in SPT, it correlated well with BAT results. Thus, based on the SPT and BAT results, this patient could be reactive to amoxicillin and ampicillin with tolerance to Pen G and V. Such a patient may have selective reactions to the R-group side chain of the amoxicillin and ampicillin which play an important role as antigenic determinant in some allergic reactions related to penicillin antibiotic.17 Nevertheless, this was not detected in our immunoassay, suggesting the limitation of the test to identify patients with allergy to side chain of the drugs.
Earlier negativization of IgE bound to basophils as compared to serum IgE antibodies was demonstrated by Fernandez et al.18 in patients allergic to amoxicillin. This might explain symptomatic patients with negative BAT results in our cohort. Our study identified four patients with positive sIgE to amoxicillin were BAT-negative. In a study by Salas et al.19 BAT negativization to amoxicillin has occurred as early as 6 months in nine of 24 patients. Drug types might contribute to variations of negativization rates. Our study also suggests that BAT negativization to amoxicillin may have occurred earlier compared to other penicillin drugs.
Our analysis involving patients with total IgE >200kU/l (n=11) revealed one BAT-positive patient with a relatively high specific to total IgE ratio ≥0.002 (0.004–0.007). It has been reported that the ratio of penicillin specific to total IgE is able to improve the diagnostic performance of immunoassay in identifying allergic patients, particularly patients with serum total IgE >200kU/l.20 An elevated specific to total IgE ratio was associated with a high level of allergen-sIgE on basophils or mast cells, whereas this is rare when the ratio is low. Thus, application of the specific to total IgE ratio may improve the overall diagnostic performance of IgE-mediated drug allergy.
One of the limitations of the study is the absence of drug provocation tests. A drug provocation tests is considered the ‘gold standard’ to confirm diagnosis of drug hypersensitivity to culprit drug. However, due to safety and ethical reasons, drug provocation tests were not performed in our study as well as in many studies. Most diagnostic studies will perform a provocation test when in vitro tests and SPTs/IDTs are negative, to reduce the risk of severe reactions.21 In this study, BAT was performed along with immunoassay based on clinical history alone. Future studies investigating the agreement of both tests should include diagnosed subjects that are positive to at least one causative drugs in intradermal test and/or drug provocation test.
ConclusionIn conclusion, the agreement between BAT and immunoassay is minimal, thus performing both tests has a minimal increase on the sensitivity of allergy diagnosis work-up for immediate reactions to penicillin. This study also suggests that SPT and specific to total IgE ratio is useful to improve diagnosis of patients allergic to penicillin antibiotics. Therefore we recommend BAT and determination of specific to total IgE ratio in cases where the diagnosis of drug allergy is highly suspect, thus avoiding the need for provocation tests.
DeclarationsEthics approval and consent to participateThis study was approved by the Medical Research of Ethics Committee of the Ministry of Health in Malaysia (KKM/NIHSEC/P13-901, NMRR-13-922-17589).
Consent for publicationInformed consent for all testing and publication of data was obtained from all patients and controls.
Availability of data and materialThe datasets used and/or analyzed during the current studies are available from the corresponding author on reasonable request.
FundingThis work was funded by the Ministry of Health, Malaysia grant JPP-IMR 13-015 NMRR-13-922-17589.
Authors’ contributionsBL conceptualized the study, performed the literature search, designed the experimental setup, performed laboratory works, contributed to analysis and interpretation of data and wrote the manuscript. FB, MMT and NA recruited patients and controls, performed SPT, contributed to analysis and interpretation of data and writing the manuscript. ZHMY contributed to the experimental setup and assisted in writing the manuscript. All authors provided critical and intellectual input during the research process. All authors read and approved the final manuscript.
Conflict of interestBL, FB, MMT, ZHMY and NA declare no competing interests.
The authors would like to thank the Director General of Health Malaysia for his permission to publish this article. The authors would also like to thank Ms. Norhidayah Yusman for her assistance in patients’ recruitment and laboratory work.