Methylenetetrahydrofolate Reductase (MTHFR) polymorphisms by impairing folate metabolism may influence the development of allergic diseases. The results of studies evaluating the relationship between MTHFR polymorphisms and atopic disease are controversial. The aim of this study was to investigate the association between the polymorphisms of C677T and A1298C for MTHFR gene and allergic rhinitis (AR) in children.
MethodsNinety patients followed up with diagnosis of allergic rhinitis in our clinic and 30 children with no allergic diseases were included in the study. All participants were genotyped for the MTHFR (C677T) and (A1298C) polymorphisms. Vitamin b12, folate and homocysteine levels were measured.
ResultsThe mean age of patients was 9.2±2.9 years; 66.7% of the patients were male. There was no significant difference between patient and control groups regarding gender, age and atopy history of the family (p>0.05). The frequency of homozygotes for MTHFR C677T polymorphism in the patient and control groups was 3.3% and 10%, respectively. The frequency of homozygotes for MTHFR A1298C polymorphism among groups was 26.7% and 16.7%, respectively. The association between allergic rhinitis and polymorphisms of C677T and A1298C for MTHFR gene was not statistically significant in patients compared with controls (p>0.05). There were no statistically significant differences between the patients and the control group in terms of serum vitamin b12, folate and homocysteine levels (p>0.05).
ConclusionWe found no evidence for an association between allergic rhinitis and polymorphisms of C677T and A1298C for MTHFR gene in children. Further studies investigating the relationship between MTHFR polymorphism and AR are required.
Allergic rhinitis (AR) is a disease developing as a result of immunoglobulin (Ig) E response to allergens in the nose and characterized by symptoms such as nasal congestion, flow, rash, and sneezing. These symptoms occur during two or more consecutive days for more than 1h on most days. Allergic rhinitis is a global health problem that causes major illness and disability worldwide. Patients from all countries, all ethnic groups, all socioeconomic conditions and of all ages suffer from allergic rhinitis. The prevalence of allergic rhinitis was found to be around 25% in the general population in Europe. Allergic rhinitis is a multifactorial disease induced by gene-environment interactions.1
Methylenetetrahydrofolate Reductase (MTHFR) is an important enzyme for folate metabolism and DNA methylation. This enzyme catalysis 5,10-methylenetetrahydrofolate's conversion to 5-methyltetrahydrofolate (5-methyl-THF). MTHFR gene is mapped on chromosome 1 at p36.3 region in humans. There are two of the well-defined single nucleotide polymorphisms (SNP) on the MTHFR gene. These are C677T and A1298C. The C677T polymorphism of MTHFR causes the alanine amino acid to convert into valine at code 222. The conversion occurs at exon 4 of MTHFR protein consisting of 656 amino acids and affects N-terminal catalytic region. In individuals with homozygote TT genotype, it is known that the enzyme activity is reduced and plasma homocysteine levels increase since homocysteine is unable to convert into methionine. The A1298C polymorphism of MTHFR results in a glu429-to-ala (E429A) substitution. Whereas the C677T transition occurs within the predicted catalytic domain of the MTHFR enzyme, the A1298C transition is located in the presumed regulatory domain. The A1298C polymorphism of MTHFR resulted in decreased MTHFR activity, which was more pronounced in the homozygous than heterozygous state.2
Lack or excess of folate which plays a role in DNA synthesis may affect gene expressions. Development and differentiation of the immune system occur through epigenetic mechanisms. Folate may change gene expression during the early development phase and therefore it may affect development of allergic phenotype.3 A diet with high methyl content during pregnancy has been shown to cause allergic asthma phenotype through epigenetic mechanisms in an animal experimental study.4 Results of studies investigating the correlation between use of folate during pregnancy and development of allergic diseases during childhood are contradictory. There are studies showing that high folate uptake during pregnancy increases allergic diseases (particularly atopic dermatitis (AD) and asthma) during childhood5–8 as well as other studies showing that high folate uptake during pregnancy does not affect.3,9–11 or decreases allergic diseases during childhood.12 In studies where polymorphism on the MTHFR gene was assessed in patients with asthma and allergic rhinitis, TT allele carrier patients were found to have asthma at a higher rate,13,14 whereas no relation was found in one of the studies.15 In those studies investigating the relationship between MTHFR and atopic diseases, mainly the relations of asthma and AD were assessed. In the study conducted only on adults (>15 years), it was assessed with respect to AR.15
In the current study, we aimed to describe the association between AR and the polymorphisms of C667T and A1298C for the MTHFR gene.
MethodsNinety patients followed up with diagnosis of allergic rhinitis in the Pediatric Immunology and Allergy clinic of Zeynep Kamil Woman's and Children's Diseases Training and Research Hospital, and 30 children with no allergic diseases were included in the study. Patients were prospectively evaluated through January 2013–June 2013. Detailed allergic disease histories of patients were obtained. Patients were inquired for age, consanguineous marriage, atopy history of the family and exposure to tobacco smoke. Presence of atopy in the family was considered positive when an allergic disease was present in first degree relatives (mother, father or sibling). The diagnosis of AR was made according to the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines.1 The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice and was approved by the local Ethics Committee of Zeynep Kamil Woman's and Children's Diseases Training and Research Hospital. Consent of all patient families was obtained for participation in the study.
Vitamin B12, folate, and homocysteine levelsBlood samples were taken after an overnight fasting, and vitamin B12, folate and homocysteine were made by using chemiluminescent immunoassay kits (Abbott Laboratories, IL, USA). The reference ranges for vitamin B12 and folate were accepted as 200–900pg/mL and 5–20ng/mL, respectively. Homocysteine levels were assessed according to normal values in Turkish children.16
MHFTR genotypingGenomic DNA was isolated from the peripheral blood samples using Magnesia DNA Blood Mini Kit (Anatolia, Turkey). C677T and A1298C of MTHFR mutations were genotyped using Bosphore Kit by Real-Time PCR with Montania 483 (Anatolia, Turkey) according to the guidelines of the manufacturer.
Statistical analysisAll results were analyzed using the SPSS (Statistical Package for the Social Sciences) 15 (SPSS Inc., Chicago, IL, USA) program. Categorical variables were described as percentages and number while continuous variables were expressed as minimum, maximum, and mean±standard deviation (SD). Student's t test was used for the comparison of normally distributed variables. Chi-square and Mann–Whitney U-tests were used for non-normally distributed variables. Spearman's correlation test was used for the correlation analyses of non-normally distributed variables. Statistical significance was defined as p<0.05.
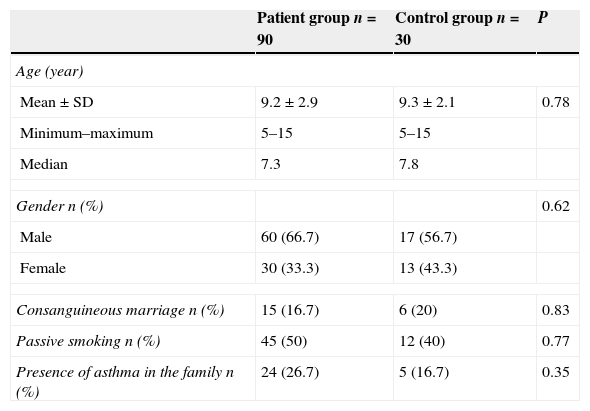
ResultsNinety children with AR and 30 healthy children, 120 children in total, were included in the study. There was no significant difference between patient and control groups with respect to gender, age, consanguineous marriage, atopy history of the family and exposure to tobacco smoke (p>0.05). Table 1 shows the comparison of groups’ sociodemographic characteristics.
Comparison of groups’ sociodemographic characteristics.
| Patient group n=90 | Control group n=30 | P | |
|---|---|---|---|
| Age (year) | |||
| Mean±SD | 9.2±2.9 | 9.3±2.1 | 0.78 |
| Minimum–maximum | 5–15 | 5–15 | |
| Median | 7.3 | 7.8 | |
| Gender n (%) | 0.62 | ||
| Male | 60 (66.7) | 17 (56.7) | |
| Female | 30 (33.3) | 13 (43.3) | |
| Consanguineous marriage n (%) | 15 (16.7) | 6 (20) | 0.83 |
| Passive smoking n (%) | 45 (50) | 12 (40) | 0.77 |
| Presence of asthma in the family n (%) | 24 (26.7) | 5 (16.7) | 0.35 |
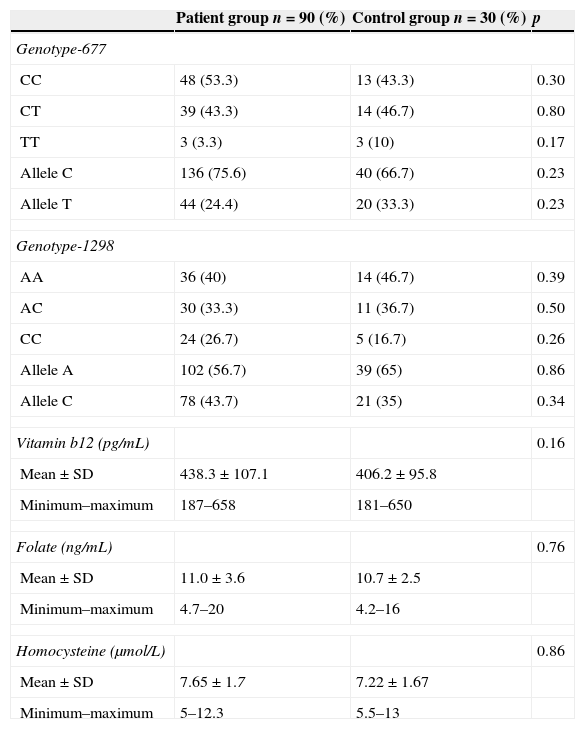
Thirty-nine (43.3%) of patients group had the CT genotype and three (3.3%) of the patients were homozygotes for MTHFR C677T polymorphism. In the control group, 14 (46.7%) were heterozygotes and three (10%) were homozygotes. The frequency of the T allele in the patients and control group was 24.4% and 33.3%, respectively. There was no statistically significant difference between genotype frequency of MTHFR C677T polymorphisms in patients compared with controls (p=0.287).
Thirty (33.3%) of the patients group had the AC genotype and twenty-four (26.7%) of the patients were homozygotes for MTHFR A1298C polymorphism. In the control group, 11 (36.7%) were heterozygotes and five (16.7%) were homozygotes. The frequency of the C allele in the patients and control group was 43.7% and 35% respectively. There was no statistically significant difference between genotype frequency of MTHFR A1298C polymorphisms in patients compared with controls (p=0.602).
The genotype and allele frequencies of the MTHFR gene C677T and A1298C polymorphisms in patients and controls are shown in Table 2.
Genotype and allele frequency of the MTHFR C677T and MTHFR A1298C polymorphism, vitamin b12, folate and homocysteine levels among patients and controls.
| Patient group n=90 (%) | Control group n=30 (%) | p | |
|---|---|---|---|
| Genotype-677 | |||
| CC | 48 (53.3) | 13 (43.3) | 0.30 |
| CT | 39 (43.3) | 14 (46.7) | 0.80 |
| TT | 3 (3.3) | 3 (10) | 0.17 |
| Allele C | 136 (75.6) | 40 (66.7) | 0.23 |
| Allele T | 44 (24.4) | 20 (33.3) | 0.23 |
| Genotype-1298 | |||
| AA | 36 (40) | 14 (46.7) | 0.39 |
| AC | 30 (33.3) | 11 (36.7) | 0.50 |
| CC | 24 (26.7) | 5 (16.7) | 0.26 |
| Allele A | 102 (56.7) | 39 (65) | 0.86 |
| Allele C | 78 (43.7) | 21 (35) | 0.34 |
| Vitamin b12 (pg/mL) | 0.16 | ||
| Mean±SD | 438.3±107.1 | 406.2±95.8 | |
| Minimum–maximum | 187–658 | 181–650 | |
| Folate (ng/mL) | 0.76 | ||
| Mean±SD | 11.0±3.6 | 10.7±2.5 | |
| Minimum–maximum | 4.7–20 | 4.2–16 | |
| Homocysteine (μmol/L) | 0.86 | ||
| Mean±SD | 7.65±1.7 | 7.22±1.67 | |
| Minimum–maximum | 5–12.3 | 5.5–13 | |
Mean vitamin b12 level was 438.3±107.1pg/mL (269–658) in the study group and 406.2±95.8ng/mL (170–650) in the control group. The difference was not statistically significant (p=0.161). Similarly, there was no statistically significant difference between the patients and the control group in terms of serum folate and homocysteine levels (respectively, p=0.757, p=0.856) (Table 2). MTHFR C677T polymorphisms were positively correlated with homocysteine levels (r: 0.372; p: 0.003).
DiscussionMTHFR mutations alter folate metabolism and reduce folate levels. The decrease in folate levels in C677T polymorphism is higher compared to A1298C.2 The association between the alteration in folate metabolism and allergic diseases in humans was initially investigated by Zou et al.13 on asthma patients. In the study where MTHFR C677T polymorphism was assessed in 433 asthma patients and 1249 healthy control subjects, TT genotype was found to be higher in patients with asthma, compared to healthy control subjects. In the study by Husemoen et al.17 assessing the factors affecting atopy and folate metabolism in adults, the association between atopy and MTHFR C67T polymorphism was determined. Atopy was found to be higher in people of TT genotype compared to other genotypes. People of TT genotype suggested that inadequate satisfaction of increased folate need that is consequent to reduced folate level may have contributed to atopy development. They stated that inhibition of the remethylation cycle consequent to folate reduction and alteration of TH1/TH2 equilibrium consequent to low antioxidant capacity may have been associated to atopy development.17 On the contrary to these studies demonstrating the positive relationship between MTHFR polymorphism and allergic diseases, Granell et al.9 did not find any relation between MTHFR polymorphism and atopy, allergy and asthma in mothers and children in their birth cohort study. In their study, atopy and asthma status of mothers was determined with a questionnaire applied during pregnancy. Asthma was determined in children according to physician diagnosis and current symptoms when children were 7–8 years old. Atopy was assessed with skin tests carried out when children were 7.5 years old. Following these contradictory results, Thuesen et al.15 investigated allergic diseases and MTHFR polymorphism in adults through a study they conducted in Denmark. In that study, atopy and bronchial hyperreactivity were assessed using specific IgE, eosinophilic cationic protein, methacholine provocation tests. AR was included in the assessment in addition to asthma. No relationship between atopic diseases (asthma, AR) and MTHFR polymorphism (C677T) was found as a result. Similarly, we were unable to find any significant difference between AR patients and control subjects in respect to MTHFR polymorphism in our study. Our difference with the study of Thuesen et al.15 was that our study was conducted on children and A1298C polymorphism was studied here as well, besides MTHFR C677T.
Studies conducted on animals have shown that high methyl donor uptake during pregnancy enhances development of allergic asthma phenotype during childhood.4 Studies evaluating the status during pregnancy when investigating the association of folate which is a methyl donor with allergic diseases were conducted as well. Results achieved in those studies have been contradictory. There are studies showing that high folate uptake during pregnancy increases allergic diseases (AD and asthma in particular) during childhood3,9–11 as well as studies showing that high folate uptake during pregnancy does not affect or decreases allergic diseases during childhood.12 The relationship between folate levels during pregnancy and asthma, AD, wheezing and respiratory tract infections was rather assessed in those studies. Development of allergic rhinitis and MTHFR polymorphisms was not investigated. The relationship between MTHFR polymorphism and atopic diseases was assessed by van der Valk et al.18 In that study, vitamin b12, folate, homocysteine levels in neonatal cord blood as well as MTHFR C677T and A1298C polymorphisms of patients were investigated. They determined that neonatal b12, folate and homocysteine levels had no effect on asthma and AD during childhood. Furthermore, MTHFR C677T homozygotes with high folate levels and MTHFR A1298C homozygotes with high homocysteine levels were shown to have higher AD development. They stated that the influence of folate and homocysteine on AD development depended on polymorphism on MTHFR gene. In that childhood study, AR development and MTHFR polymorphism were not investigated. We did not find statistically significant differences between the patients and the control group in terms of MTHFR polymorphism, folate, vitamin B12, and homocysteine levels.
The limited participant number was the limiting aspect of our study. However, being the first study investigating AR and MTHFR polymorphism during childhood is quite important.
In conclusion, we did not find any significant difference between patients with allergic rhinitis and control subjects in respect to MTHFR polymorphism, folate, vitamin B12, and homocysteine levels. Further studies investigating the relationship between MTHFR polymorphism and AR are required.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.