Atopic dermatitis (AD) is an eczematous skin disease. Our aim was to evaluate the clinical and laboratory findings of children with AD and identify the higher responsive group to moisturizers.
Materials and methodsTotal and specific IgE, eosinophil count, prick/patch test results of patients with AD were retrospectively analyzed. The presentation SCORAD was compared between the demographic and clinical subgroups. The SCORAD change (presentation to third month) between the intrinsic and extrinsic groups was compared. The effect of age, sex, disease duration, presentation SCORAD, being intrinsic/extrinsic, exclusive breastfeeding duration, familial atopy, total IgE, eosinophil count, concomitant illness presence, moisturizer use frequency and exacerbation frequency on SCORAD change was examined.
ResultsThe mean age was 3.65±3.77 years. Food allergy was found in 5.90% and inhalant allergy was found in 12.67% of patients. 158 (44.5%) were mild, 154 (43.4%) were moderate and 43 (12.1%) were severe AD. 141 (39.7%) were intrinsic AD. The SCORAD at 3rd visit and SCORAD change was different between the intrinsic and extrinsic groups. SCORAD change was positively associated with presentation SCORAD, eosinophil count, moisturizer use frequency and being extrinsic AD.
ConclusionsThe clinical and laboratory findings of AD patients in our community were revealed. Higher SCORAD and eosinophils at presentation, frequent daily moisturizer use and being extrinsic increased the moisturizer response. Although the barrier defect was shown to be lesser in intrinsic AD by considering transepidermal water loss, this study is the first to evaluate intrinsic and extrinsic AD patients according to response to moisturizers.
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by itching and eczematous lesions. The prevalence in children is known to be about 15%.1
AD pathogenesis is multifactorial, and is involved with genetic susceptibility, skin barrier defects, environmental factors and immunologic dysregulation. According to the outside-in theory, the disorder in the epidermal barrier initiates the disease, followed by immunological changes. In contrast, according to the inside-out theory, the immune dysregulation is the primary cause of the disease, and the barrier changes are an epiphenomenon.2
Food and inhalant allergens are associated with AD exacerbations. The prevalence of food allergy in AD is estimated to be 30%. Hen's egg, cow's milk, wheat, soy, shellfish, fish, and peanut are the most common offending foods for food allergy in AD. There are conflicting data regarding whether inhalant allergens have the role through direct contact with the skin, or inhalation and absorption through the respiratory tract.3,4
ScoringAD (SCORAD) is a frequently used index that evaluates objective and subjective symptoms of AD to assess the extent and severity of the condition. This index is used to assess disease severity as well as to evaluate treatment response in follow-up.5
AD can be divided into the extrinsic and intrinsic types. Intrinsic AD differs from extrinsic AD in terms of cytokines, and intrinsic AD barrier defects are mild in comparison to extrinsic AD.6,7
Moisturizers are safe and effective first line therapies of AD during flares and remission. Their widely known effect is a moisturization effect. They have also effects on skin microbiome by reducing Staphylococcus aureus colonization, decreasing transepidermal water loss (TEWL), upregulating antimicrobial proteins (AMP) and terminal differentiation proteins’. Therefore, moisturizers are the cornerstone of AD treatment.8,9 Moisturizers have been shown to decrease SCORAD scores, increase relapse free times, and they have steroid sparing effects.10,11
As AD pathogenesis contains various complex mechanisms, the clinical features vary by age, race and the population.12 The aim of this study was to investigate the clinical and laboratory findings of Turkish children with AD, and to evaluate the response to daily emollient therapy in different phenotypes of AD.
Materials and method355 patients (range: 1 month to 17 years of age), who presented to our department between January 2016 and January 2017, and who were diagnosed with AD according to the Hanifin Rajka diagnostic criteria were included in the study.13 The study was approved by the local ethical committee.
Patients with other acute and chronic infectious and inflammatory diseases, and who were on systemic or topical corticosteroid therapy in the previous month of admission were excluded from the study. All of the patients included in the study were patients who did not receive any kind of treatment for AD before their enrolment in the study.
Age, gender, familial atopy, exclusively breastfeeding duration (<4 months/≥4 months) were recorded from the patient files. Familial atopy was defined as a positive response to the question “Has the father or the mother ever suffered from asthma, rhinitis or eczema?”.
The laboratory results of the patients, i.e. complete blood count (CBC), food specific IgE (fx5), inhalant allergen specific IgE (Phadiotop), and total IgE, were retrospectively examined. In our clinic, CBC is routinely analyzed with The Mindray BC-6800 hematology analyzer (Mindray Bio-Medical Electronics Co., Ltd, Shenzhen, China). Total IgE and specific IgE levels are routinely analyzed by the ELISA method.
Disease duration was specified as the time between the first complaint of the disease and the time of presentation to our clinic.
Skin prick test (SPT) results of all patients were obtained from their health records. SPT was applied with the same technique to all patients admitted to our clinic. SPT was applied to the forearm or back of the patient. Histamine (10mg/ml) and physiological saline were used as positive and negative references. Reactions were evaluated 15minutes after the application. A reaction of at least 3mm, or larger than the negative control was considered to be positive for the test. Common aeroallergens were used in SPT (Dermatophagoides pteronyssinus, Dermatophagoides farinae, grasses mix, cereals mix, trees mix, weed-mix, cockroaches, cat and dog dander, cow's milk, hen's egg, wheat and soy) (ALK – Abello SPT kit).
The atopy patch test (APT) results were also recorded from patient files. In our clinic we perform APT as follows: One drop (50mL) each of fresh cow's milk containing 3.5% fat, whisked egg, wheat flour dissolved in distilled water (1g/10mL), and soy bean flour was put on filter paper and applied to the uninvolved skin of the child's back with IQ chamber (Chemotechnique Diagnostics). Distilled water was used as negative control. Application sites were checked after 20min for immediate reactions. The results after 48-h occlusion time were recorded 20min after removal of the cups to avoid false-positive results caused by an irritant effect. The final evaluation of the test was done 24h afterwards. Reactions were evaluated according to the European Task Force on AD.14
Patients were grouped according to their SCORAD scores at presentation. Patients who scored <25 points were grouped as mild, 25–50 points were grouped as moderate, and >50 points were grouped as severe AD. From patient files and monthly follow-ups, the frequency of use of recommended moisturizer treatment per day was obtained. In our clinic all of the patients are routinely advised to use emollients twice a day at least five days per week.
AD patients were also grouped as intrinsic and extrinsic. Patients with high total IgE and/or positive food or inhalant specific IgE and/or having other atopic diseases were considered as extrinsic AD. The patients with low total IgE, and negative food and inhalant specific IgE and no other atopic diseases were considered as intrinsic AD groups.15
The SCORAD scores of patients at 1st, 2nd and 3rd controls were recorded and compared. The difference between the SCORAD scores at presentation and the last control was defined as SCORAD change. The impact of age, gender, familial atopy, SCORAD score at presentation, intrinsic/extrinsic AD phenotype, exclusively breastfeeding duration, total IgE and eosinophil counts, number of exacerbations between the presentation and the 3rd month, and the frequency of daily moisturizer use on SCORAD change was examined by linear regression analysis. In this analysis pollen and mold allergic patients were excluded because of the possible effect of the seasonal change on the SCORAD, food allergic patients were excluded because of the effect of elimination diets on SCORAD, patients who have mite allergy and who performed one or more of the house dust mite avoidance strategies given routinely to all house dust mite allergic patients were also excluded from the study. Cat/dog allergic patients were not excluded from the study because none of them had an animal in their houses, and none of them performed avoidance strategies from these animals. Therefore, the linear regression analysis was performed on 316 of the 355 AD patients.
Statistical analysesResults were given as either mean±standard deviation (SD) or as median±interquartile range (IQR) according to the distribution. Student's t-test and Mann–Whitney U-test were used for the comparison of normally and non-normally distributed variables, respectively. Pearson's and Spearman's correlations were used. Linear regression analysis was used to investigate the effect of clinical, laboratory and demographical variables on SCORAD change. p<0.05 was considered to be statistically significant.
ResultsThere were 355 patients in the study. The mean age of the patients was 3.65±3.77 years. 192 (54.08%) of the patients were male, and 163 (45.91%) were female. 162 patients (45.63%) were two years of age and younger. Familial atopy was present in 74 patients (20.8%).
134 patients (37.74%) were exclusively breastfed for less than four months, and 208 were exclusively breastfed (58.59%) for four months or more.
According to the patients’ history, 69 patients (19.4%) had an allergic disease other than AD. These diseases were wheezing/asthma in 56 patients, allergic rhinitis in seven patients, drug hypersensitivity in three patients, urticaria in two patients, and anaphylaxis in one patient.
The median disease duration was 8.0 months (4.0–13.0). The median total IgE value was 40.10 IU/mL (12.30–140), the median absolute eosinophil count was 340.0 (170.0–510.0)·109/L at presentation.
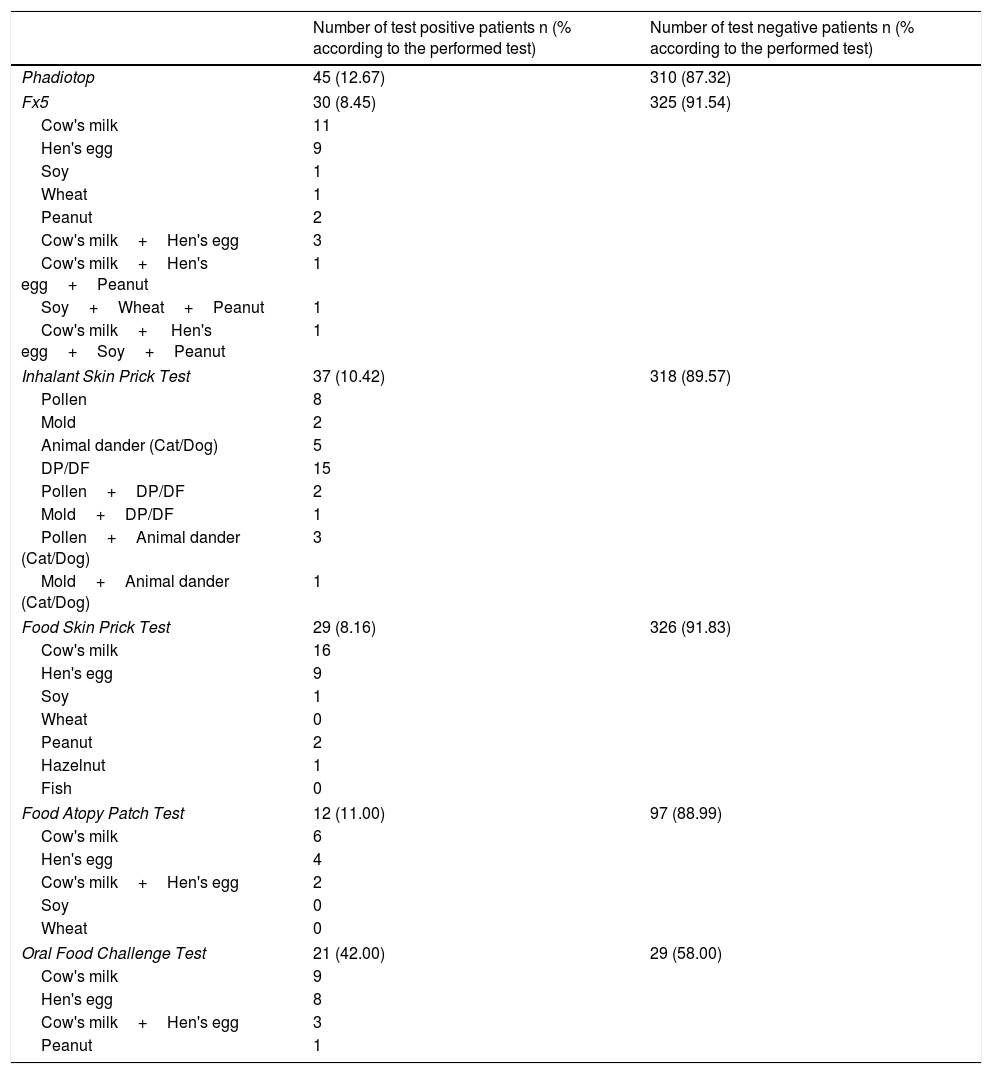
Fx5 was positive in 30 (8.45%) patients, and negative in 325 (91.54%) patients. The subgroups of fx5 are presented in Table 1. Food SPT was performed on all of the AD patients; the results are presented in Table 1. Food APT was performed on the patients with positive food SPT and/or positive food specific IgE and other randomly selected 72 patients. A total of 109 food APTs were performed. The results are presented in Table 1.
Specific IgE, skin prick test, food atopy patch test and oral food challenge test results of AD patients.
| Number of test positive patients n (% according to the performed test) | Number of test negative patients n (% according to the performed test) | |
|---|---|---|
| Phadiotop | 45 (12.67) | 310 (87.32) |
| Fx5 | 30 (8.45) | 325 (91.54) |
| Cow's milk | 11 | |
| Hen's egg | 9 | |
| Soy | 1 | |
| Wheat | 1 | |
| Peanut | 2 | |
| Cow's milk+Hen's egg | 3 | |
| Cow's milk+Hen's egg+Peanut | 1 | |
| Soy+Wheat+Peanut | 1 | |
| Cow's milk+ Hen's egg+Soy+Peanut | 1 | |
| Inhalant Skin Prick Test | 37 (10.42) | 318 (89.57) |
| Pollen | 8 | |
| Mold | 2 | |
| Animal dander (Cat/Dog) | 5 | |
| DP/DF | 15 | |
| Pollen+DP/DF | 2 | |
| Mold+DP/DF | 1 | |
| Pollen+Animal dander (Cat/Dog) | 3 | |
| Mold+Animal dander (Cat/Dog) | 1 | |
| Food Skin Prick Test | 29 (8.16) | 326 (91.83) |
| Cow's milk | 16 | |
| Hen's egg | 9 | |
| Soy | 1 | |
| Wheat | 0 | |
| Peanut | 2 | |
| Hazelnut | 1 | |
| Fish | 0 | |
| Food Atopy Patch Test | 12 (11.00) | 97 (88.99) |
| Cow's milk | 6 | |
| Hen's egg | 4 | |
| Cow's milk+Hen's egg | 2 | |
| Soy | 0 | |
| Wheat | 0 | |
| Oral Food Challenge Test | 21 (42.00) | 29 (58.00) |
| Cow's milk | 9 | |
| Hen's egg | 8 | |
| Cow's milk+Hen's egg | 3 | |
| Peanut | 1 | |
Oral food challenge (OFC) was positive in 21 out of 50 patients to whom OFC was performed. 5.91% of all the AD patients had food allergy (Table 1).
Phadiotop was negative in 310 patients (87.32%), and positive in 45 patients (12.67%) (Table 1). Inhalant allergen SPT results are presented in Table 1.
141 patients (39.71%) were grouped as intrinsic AD, and 214 patients (60.28%) were grouped as extrinsic AD.
According to the records, 237 patients (66.76%) used emollients once daily at least five days a week, 118 patients (33.23%) used them twice daily at least five days week. The files of the patients revealed that if the patients were in an acute exacerbation of eczema, they were offered low potency topical corticosteroids according to the World Health organization (WHO) corticosteroid potency classification (group VII) for up to seven days, and that none of the patients were prescribed immunomodulators or oral antihistaminergic drugs.
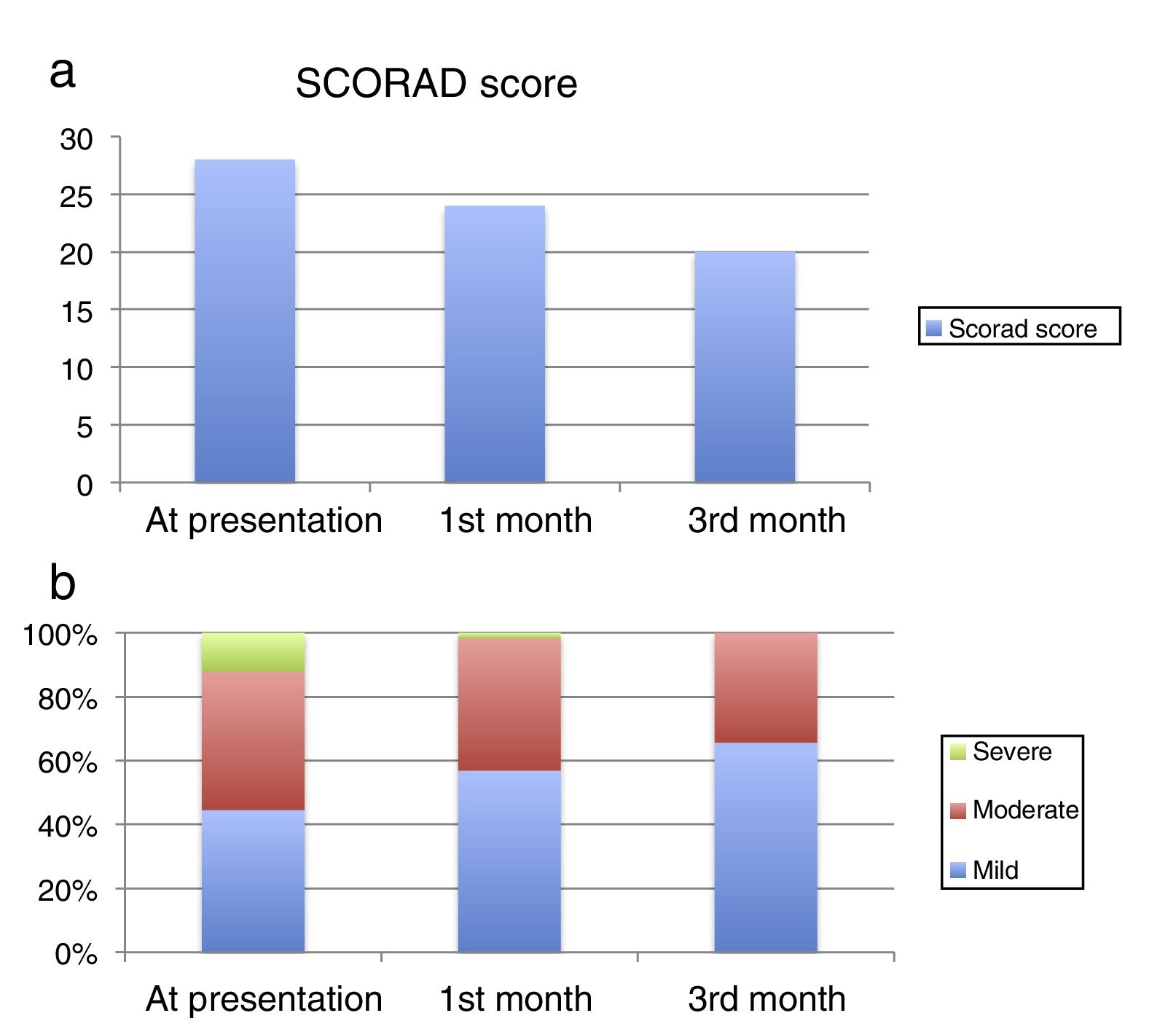
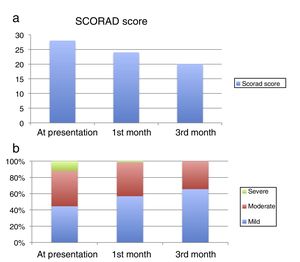
The median SCORAD score was 28 (20–38) at presentation, 24 (16–33) at the 1st month visit, and 20 (13–30) at the 3rd month visit (Fig. 1a). The median values were statistically significantly different between the visits (p<0.001). At presentation, 158 patients (44.5%) had mild, 154 patients had moderate (43.4%) and 43 patients (12.1%) had severe AD according to their SCORAD scores. At the 1st month visit, 202 patients (56.9%) had mild, 147 patients (41.4%) had moderate and six patients (1.69%) had severe AD. At the 3rd month visit, 233 patients (65.63%) had mild, and 122 patients (34.36%) had moderate AD (Figure 1b).
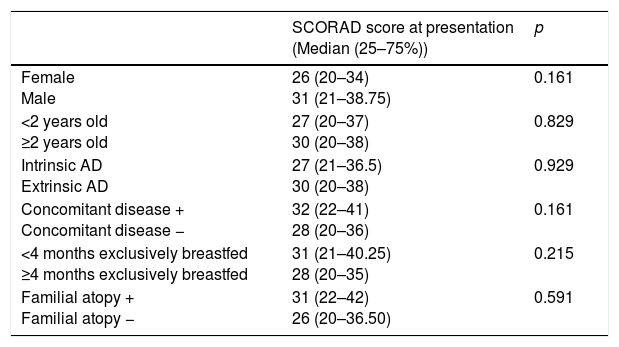
The comparison of the median SCORAD scores according to the gender, age group, intrinsic versus extrinsic AD, existence of concomitant disease, duration of exclusively breastfeeding, and familial atopy are presented in Table 2. There were no differences between the groups according to the median SCORAD scores at presentation.
Comparison of the median SCORAD scores according to the different clinical and demographic variables.
| SCORAD score at presentation (Median (25–75%)) | p | |
|---|---|---|
| Female Male | 26 (20–34) 31 (21–38.75) | 0.161 |
| <2 years old ≥2 years old | 27 (20–37) 30 (20–38) | 0.829 |
| Intrinsic AD Extrinsic AD | 27 (21–36.5) 30 (20–38) | 0.929 |
| Concomitant disease + Concomitant disease − | 32 (22–41) 28 (20–36) | 0.161 |
| <4 months exclusively breastfed ≥4 months exclusively breastfed | 31 (21–40.25) 28 (20–35) | 0.215 |
| Familial atopy + Familial atopy − | 31 (22–42) 26 (20–36.50) | 0.591 |
There were no significant correlations between the SCORAD score at presentation and the disease duration, total IgE value (p=0.948, rho=0.003; p=0.183, rho=0.071, respectively). There was a significant positive correlation between SCORAD score at presentation and absolute eosinophil count (p=0.002, rho=0.160).
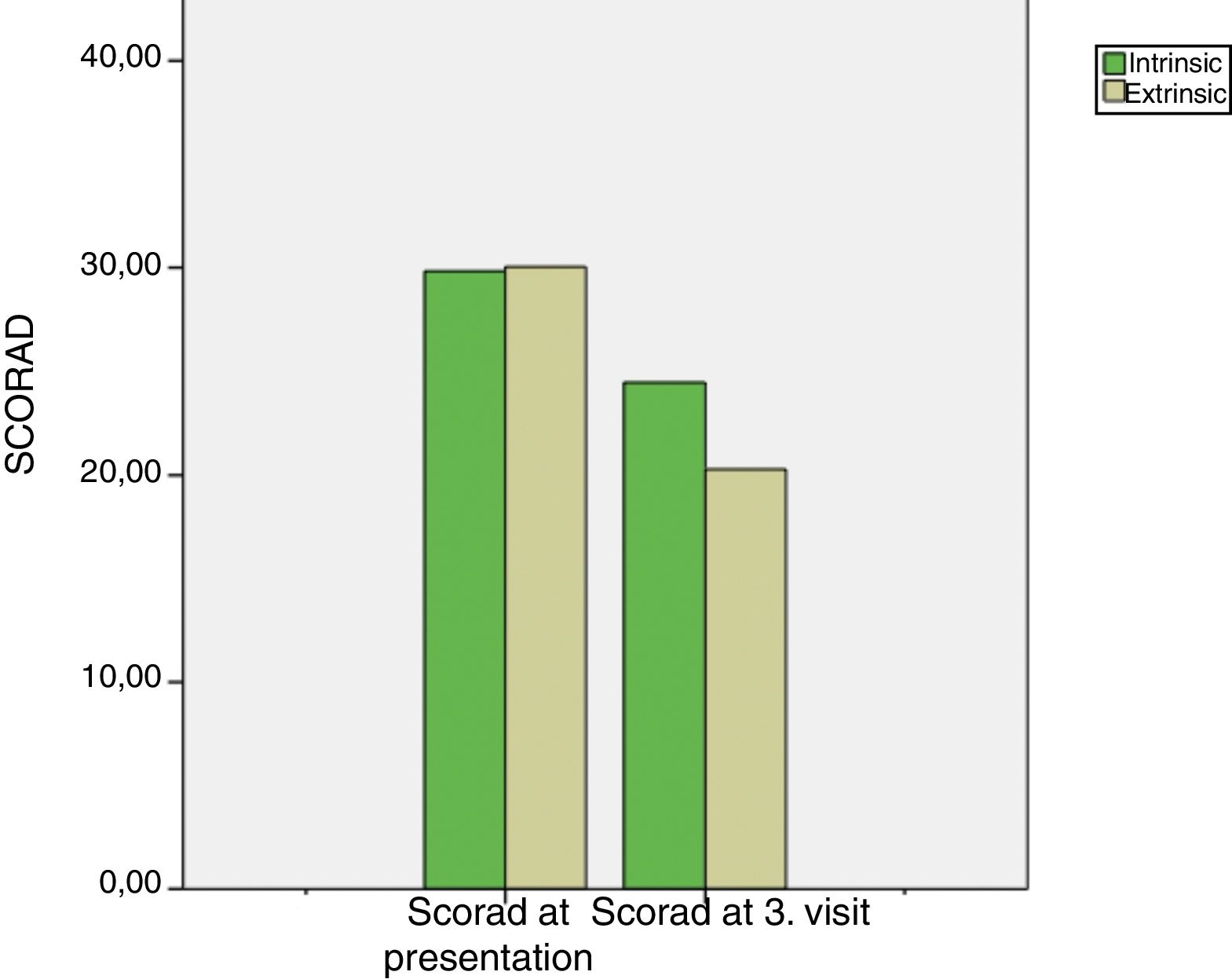
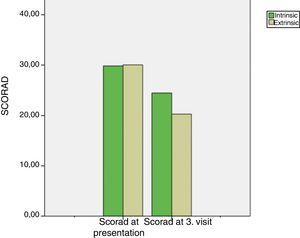
Intrinsic AD group's median SCORAD score at the 3rd month visit was 22 (16.5–30) and extrinsic AD group's median SCORAD score at the 3rd month visit was 20 (11–29.25). Both the intrinsic and extrinsic groups’ SCORAD scores significantly differed between the presentation and the 3rd month visits (p<0.001, for both) (Fig. 2). Moreover, there was a significant difference between the intrinsic and extrinsic AD groups’ 3rd month's SCORAD scores.
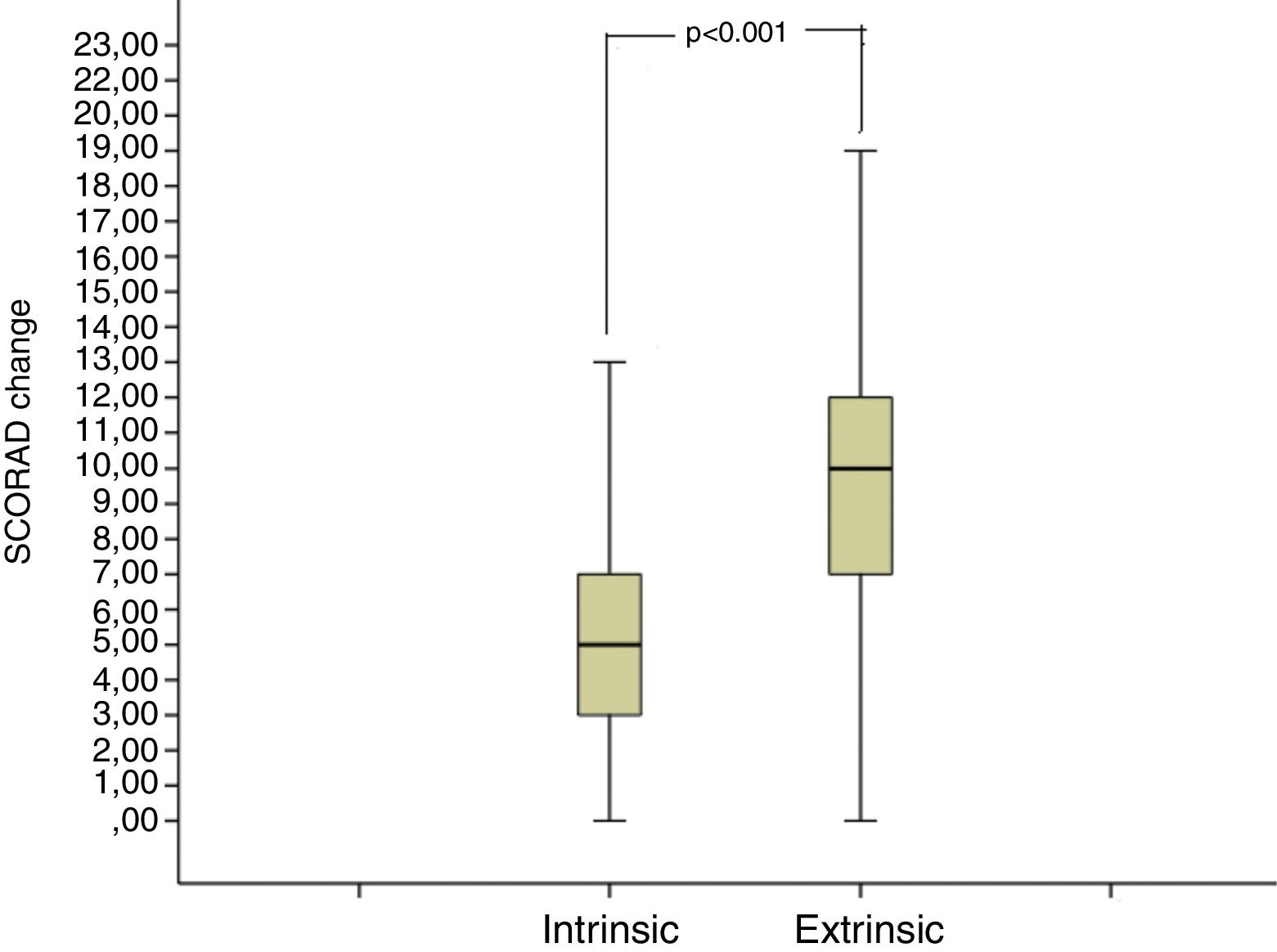
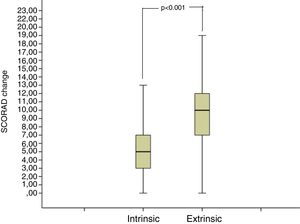
The median SCORAD change value of the intrinsic AD group was 5 (3–7), and the median SCORAD change value of the extrinsic AD group was 10 (7–12.25). The SCORAD change value was significantly different between the intrinsic and the extrinsic AD groups (p<0.001) (Fig. 3).
The median frequency of acute flares was four (3–5) in the three months before presentation, and two (1–3) during the three-month follow-up. There was a statistically significant difference between the number of acute flares between these time periods (p<0.001).
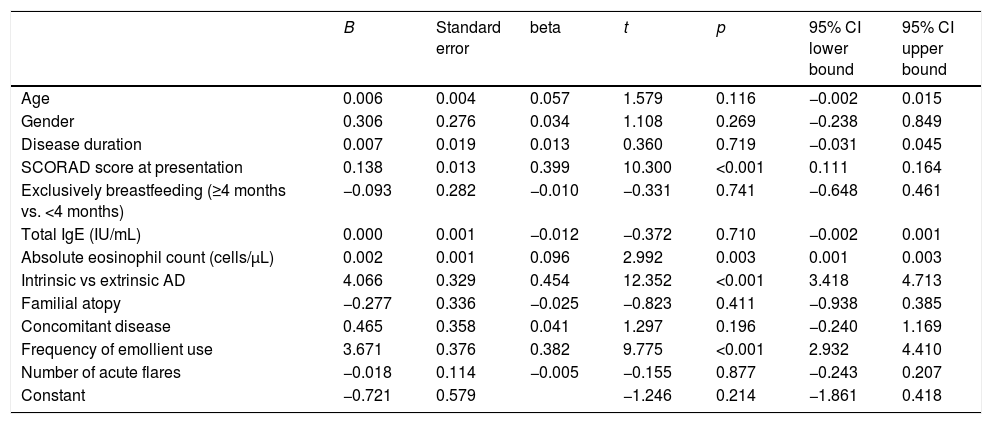
The linear regression analysis demonstrated that SCORAD change was positively associated with higher eosinophil count, higher SCORAD score at presentation, twice daily emollient use, and extrinsic AD phenotype (Table 3).
Regression analysis of the effect of the different variables to the SCORAD change value.
| B | Standard error | beta | t | p | 95% CI lower bound | 95% CI upper bound | |
|---|---|---|---|---|---|---|---|
| Age | 0.006 | 0.004 | 0.057 | 1.579 | 0.116 | −0.002 | 0.015 |
| Gender | 0.306 | 0.276 | 0.034 | 1.108 | 0.269 | −0.238 | 0.849 |
| Disease duration | 0.007 | 0.019 | 0.013 | 0.360 | 0.719 | −0.031 | 0.045 |
| SCORAD score at presentation | 0.138 | 0.013 | 0.399 | 10.300 | <0.001 | 0.111 | 0.164 |
| Exclusively breastfeeding (≥4 months vs. <4 months) | −0.093 | 0.282 | −0.010 | −0.331 | 0.741 | −0.648 | 0.461 |
| Total IgE (IU/mL) | 0.000 | 0.001 | −0.012 | −0.372 | 0.710 | −0.002 | 0.001 |
| Absolute eosinophil count (cells/μL) | 0.002 | 0.001 | 0.096 | 2.992 | 0.003 | 0.001 | 0.003 |
| Intrinsic vs extrinsic AD | 4.066 | 0.329 | 0.454 | 12.352 | <0.001 | 3.418 | 4.713 |
| Familial atopy | −0.277 | 0.336 | −0.025 | −0.823 | 0.411 | −0.938 | 0.385 |
| Concomitant disease | 0.465 | 0.358 | 0.041 | 1.297 | 0.196 | −0.240 | 1.169 |
| Frequency of emollient use | 3.671 | 0.376 | 0.382 | 9.775 | <0.001 | 2.932 | 4.410 |
| Number of acute flares | −0.018 | 0.114 | −0.005 | −0.155 | 0.877 | −0.243 | 0.207 |
| Constant | −0.721 | 0.579 | −1.246 | 0.214 | −1.861 | 0.418 |
The aim of this study was to establish the clinical and laboratory findings of patients with AD, and to evaluate the response to moisturizer treatment according to the phenotypes. The results of the study revealed the clinical and laboratory findings, and allergen sensitivities of children with AD, and the reduction in SCORAD score with maintenance moisturizing treatment has been shown to be associated with extrinsic AD phenotype, higher eosinophil count, higher SCORAD score at presentation, and twice daily use of moisturizer, where each factor was independent of the others.
Some studies in the literature demonstrated that factors such as age, sex, exclusively breastfeeding duration, and concurrent allergic diseases affected the severity of AD.12,16 In the current study, different demographic groups exhibited similar severity of AD.
Many aeroallergens and food allergens are considered to be triggering factors in AD patients. In one study, aeroallergen sensitivity was found to be 80%.3 In the current study, house dust mite sensitivity was the most commonly encountered allergen among the aeroallergens, which was in accordance with the literature.12 Around one third of patients with AD have been reported to have food allergies in various studies.4,17 In the current study, 5% of the patients were found to have food allergies. In the selection of patients to perform OFC, both the APT positivity and/or the SPT positivity were used. Therefore, this selection procedure allowed to detect most of the food allergic patients. Yet, since APT was not applied to all patients, this may have led to not detecting some cases of food allergy.
Studies have shown that the eosinophil count and total IgE level were positively correlated with SCORAD.18,19 In the current study, although there was a positive correlation between eosinophil counts and SCORAD score in accordance with the literature, total IgE level was not correlated with SCORAD score. This discrepancy with the literature may be due to the fact the patients with intrinsic AD (consisting of patients with low total IgE) were relatively more compared to the other studies. The contribution of our study to the literature was to investigate the effect of eosinophil and total IgE values at presentation on SCORAD changes. In this regard, the impact of the eosinophil value at presentation on moisturizer response independent of other factors was demonstrated.
AD patients may be classified into intrinsic and extrinsic subgroups. The prevalence of intrinsic AD has been shown to be 10–45%.20,21 Some studies reported that intrinsic AD was a milder disease,22 some reported similar disease severity in both.23 In the current study, intrinsic AD patients accounted for approximately 40% of the patients, and the disease severity of them at presentation was not different from the extrinsic group. The lack of a clear consensus on intrinsic and extrinsic AD definitions, and the different cut-off values for total IgE levels used in the differentiation of intrinsic and extrinsic AD in different studies may have led to these inconsistencies in outcomes.
Moisturizer treatment is effective in both flare-ups and maintenance treatment of AD, and was shown to reduce both the attack frequency and the severity of the disease.24 Moisturizers that are routinely recommended in our clinic are sodium lauryl sulphate and urea free moisturizers, which might cause a burning sensation, stinging and might have irritant effects. From the patients’ files, even if all the patients did not use the same moisturizers, it was observed that all patients were using one of the moisturizers containing humectant, occlusive and emollient agents. Although there is no clear consensus on how many times a day moisturizers should be used in AD, it was shown in our study that using moisturizers twice a day at least five days a week increased the SCORAD change, and this shows that this frequency reduced the disease severity. As expected, the disease severity decreased with maintenance moisturizer treatment in our patients. According to our results, there was a positive association with SCORAD change and higher SCORAD scores at presentation, and higher eosinophil counts, irrespective of other factors. This result may be due to the association of eosinophil count with disease severity, and the higher response to barrier healing therapy with the increasing barrier defect, which is also associated with the disease severity. According to our results, another factor increasing the SCORAD change irrespective of other factors was having an extrinsic AD phenotype. In two studies in the literature, extrinsic AD patients were found to have higher transepidermal water loss (TEWL) and less skin hydration compared to intrinsic AD patients and the controls. According to the results of these studies, it was concluded that the barrier defect in intrinsic AD was less than extrinsic AD.7,25 An improved barrier profile in intrinsic AD was reported to be due to a higher Th17 profile (IL-17 induces beta-defensin-2 expression)26,27 and a lower frequency of filaggrin mutations.28 In our study, the SCORAD change value was more in the extrinsic AD group, which might be interpreted as moisturizer treatment being more beneficial to extrinsic AD patients than intrinsic AD patients. Although the initial SCORAD scores were similar in both groups, the effect of moisturizing treatment, which was the effective treatment for skin barrier improvement, might be less due to the lack of barrier defects in intrinsic AD patients. In the literature, no other study has been found evaluating the barrier defect differences in intrinsic and extrinsic AD groups by investigating moisturizer treatment response of the patients, and our study is the first to evaluate from this point of view.
The limitations of our study are: (1) The APT was not applied to all the patients, so some patients with food allergies might not have been detected. (2) Because of the retrospective nature of the study, there was no control group and the frequency of moisturizer use per day was obtained from patients’ history and thus incorrect patient history might be possible. (3) Objective measures such as TEWL and capacitance were not obtained, and only clinical assessments were used for assessing treatment responses. (4) Although the SCORAD scores of the patients at the third month of the study who were offered corticosteroids might have been affected by corticosteroid usage for AD exacerbations, in the final regression analyses, since the number of eczema exacerbations was included as a confounder, the usage of corticosteroids was controlled for, to a certain extent. (5) There are natural cyclical changes of AD and this might have affected the SCORAD scores.
The strengths of our study are: (1) Specific IgE, total IgE and SPT results of all patients could be evaluated. (2) The SCORAD scores of the patients were recorded at more than one visit, and the disease severity and moisturizer treatment response could be assessed with this objective scoring. (3) In the analysis of factors impacting SCORAD change value, exclusion of patients whose 3rd visit's SCORAD scores might have been affected by allergen avoidance strategies increased the reliability of our analysis.
In conclusion, in this study, the clinical and laboratory findings of children with AD in our community were reported. The group with the highest response to moisturizers was shown to be extrinsic AD patients, the patients with higher SCORAD scores at presentation and the patients with higher eosinophil count. This study is important because it is the first study to evaluate intrinsic and extrinsic AD patients according to their response to moisturizers, and new studies are required to be undertaken about this subject.
Conflict of interestThe authors involved in this study have nothing to disclose regarding funding or any conflicts of interest with respect to this manuscript.