The aim of the present study is to assess whether a single determination of the fraction of exhaled nitric oxide (FENO), added to the measurements usually taken during a routine checkup, helps in the prediction of the recurrence of asthma attacks in controlled patients who are not receiving any baseline treatment; and whether or not treatment of the said latent inflammation is appropriate.
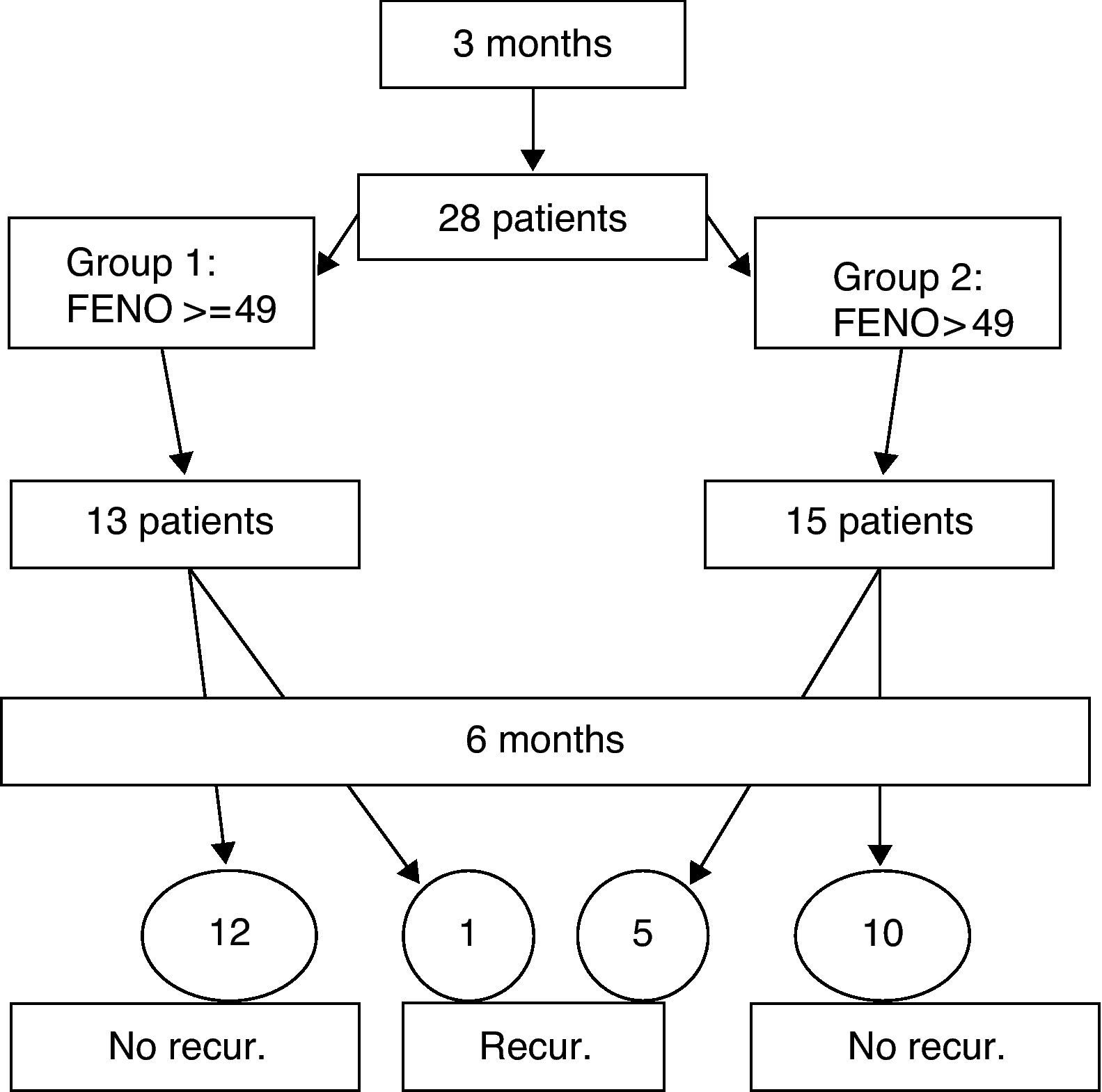
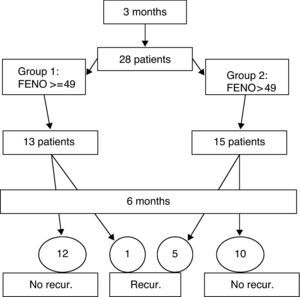
MethodsObservational study of prospective cohorts. Over a period of three months, data was collected from 28 patients (6 to 14 years) who met the conditions of the inclusion criteria, with a follow up appointment after six months.
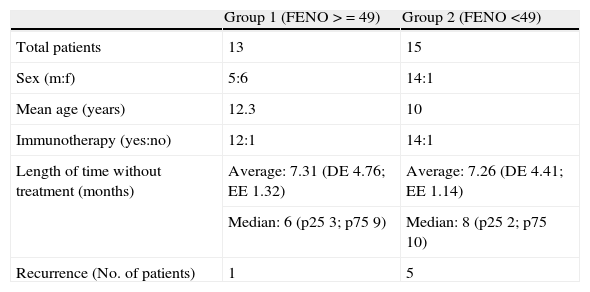
ResultsPatients were divided into two groups: 13 with FENO values of 49 and over, and 15 with values of under 49. Five patients in the subgroup with low FENO values suffered recurrence, in contrast to only one in the group with high values. The positive predictive value of the high values of the FENO was 7.69, with no significant differences between the two groups.
ConclusionsCertain doubts were raised about the usefulness of the FENO, as opposed to the traditional methods of asthma control with regard to the therapeutic management of clinically controlled patients who are not receiving treatment and who have high FENO values. It would appear unwise to recommend the systematic treatment of patients with high FENO values, when measured during a routine check-up, in cases of asthma with an allergic component and are asymptomatic or in a phase of asthma under good control.
Measuring the fraction of exhaled nitric oxide (FENO) is a quick, simple and economical way of measuring the level of eosinofilic inflammation of the airways,1–3 especially since the introduction of new, portable measuring devices. It has meant a significant advance in the management of outpatients suffering from asthma with an allergic component, in that it allows for more precise monitoring of the inflammation and adjustment of treatment. It has also led to a significant advance in the diagnosis of patients whose asthma is allergen-induced. It has been endorsed and validated by a number of studies,4–8 both as a way of diagnosing and of managing such patients. Nevertheless, doubts have been raised about the interpretation of the measurement in certain situations, such as the therapeutic management of high FENO values in clinically controlled patients.9–11
The aim of this study is to find out if a single measurement of the FENO, added to the measurements usually taken during a routine checkup at a specialised clinic, helps in the prediction of the recurrence of asthma attacks in asymptomatic patients diagnosed with asthma with an allergic component and allergic rhinitis, who are not receiving either baseline treatment or corticosteroids or antileukotrienes. We also analyse whether or not treatment of the said latent inflammation is appropriate.
Patients and methodsAn observational study of prospective cohorts. Data was collected over a period of three months from patients who attended our clinic and who met the conditions of the inclusion criteria shown in Table 1. Controlled asthma is defined according to the criteria of the PRACTALL consensus,12 and recurrence as loss of control according to the same criteria. Record was made of clinical data, pulmonary function, and the FENO by means of the NIOX-MINO ® (Aerocrine AB, Sweden) monitor, a device that has demonstrated its correlation with traditional devices, and the validity of its measurements.13,14 No criteria was provided for baseline treatment, and patients were evaluated a second time six months after their first visit, when further records were taken of the clinical data, respiratory function, and the FENO. The following variables were studied: the FENO value on the first visit; age; sex; the specific allergenic immunology treatment during the observation period; the length of time without baseline treatment prior to the first visit; and the existence, or otherwise, of recurrence. For the statistical analysis, Fisher's exact test was used, and the data were analysed using the EPIDAT 3.1 statistics programme.
Inclusion criteria.
| •Age: 6-14 years |
| •Diagnosis by specialist in asthma and allergic rhinitis |
| •Controlled asthma |
| •Normal respiratory function. FEV1 >80%. MEF 50 >70%. FEV1/VC >80%. |
| •No baseline treatment for asthma (oral or inhaled corticosteroids, bronchodilators, chromones or antileukotrines) for at least 30 days prior to visit. |
| •No symptoms, at time of measurement, of rhinitis, asthma or respiratory infection. |
Groups.
| Group 1 (FENO >=49) | Group 2 (FENO <49) | |
| Total patients | 13 | 15 |
| Sex (m:f) | 5:6 | 14:1 |
| Mean age (years) | 12.3 | 10 |
| Immunotherapy (yes:no) | 12:1 | 14:1 |
| Length of time without treatment (months) | Average: 7.31 (DE 4.76; EE 1.32) | Average: 7.26 (DE 4.41; EE 1.14) |
| Median: 6 (p25 3; p75 9) | Median: 8 (p25 2; p75 10) | |
| Recurrence (No. of patients) | 1 | 5 |
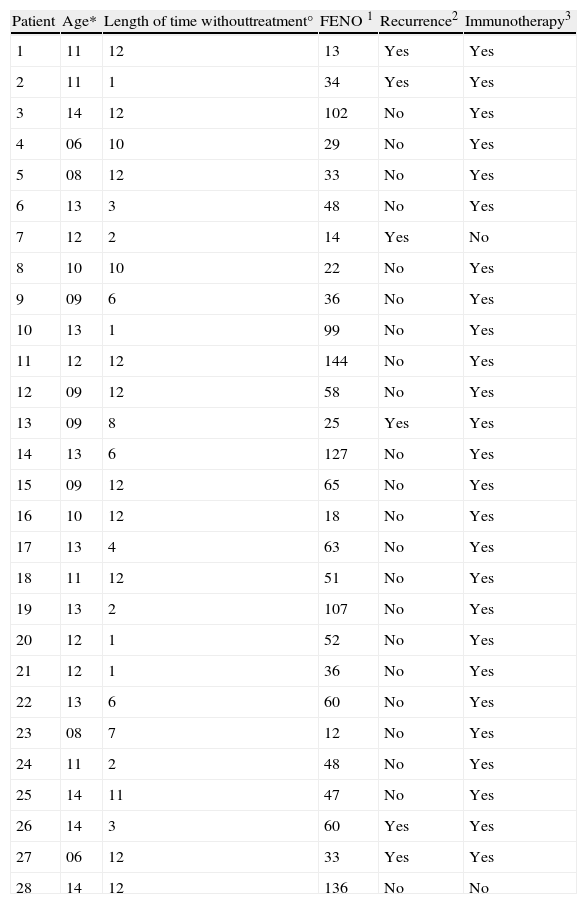
The study was of 28 patients, 21 males and 7 females, with a mean age of 11.1 years (range 6-14 years) who met the conditions of the inclusion criteria, over a period of three months. The design is shown in Fig. 1 and the full results are in Table 3. The median of the FENO values was 48 ppb, with values at p25 of 30 and at p75 of 57 ppb, and a range of 12-144 ppb The sample was divided into two subgroups, according to the FENO values and taking as a reference the value of 49 ppb This is cited as a value above which it is possible to predict greater risk of recurrence,15 and which is practically the same as the median value obtained in our study (data which may be seen as conferring greater validity on this subdivision). Thus, Group 1 consisted of those patients with a figure equal to, or higher than, 49 ppb, and Group 2 of those with figures lower than 49 ppb. The characteristics and data of each group are shown in Table 2. The groups can be mutually compared in terms of treatment with specific allergenic immunotherapy (all patients except one in both groups) and in terms of age and length of time without treatment. With regard to sex, there is a difference between the two groups, although this would not appear, a priori, to influence the results.4 Appointments were made to see the patients again six months after their first visit, with no guidelines for baseline treatment being given in any case (bearing in mind the fact that the patients were asymptomatic and in a phase of controlled asthma). Clinical data and pulmonary function using spirometry were again recorded. The latter was normal in all patients. In Group 1 (figures equal to, or higher than, 49 ppb), only one patient suffered recurrence. In Group 2, in contrast, five patients suffered recurrence. On the basis of this data, the value of a high level (above 49 ppb) of the FENO to predict recurrence, i.e., the positive predictive value was 7.69, and the negative predictive value was 66.67. A sensitivity of 16.67% and a specificity of 45.45% were obtained. The relative risk was 0.2 (confidence interval 95%: 0.03-1.72). Fisher's exact test offered a value of p=0.1166, which leads us to conclude that there is no significant difference between the groups. Finally, of the six patients who suffered recurrence, three showed FENO values equal to, or lower than, 25 ppb, out of a total of six patients who showed figures lower than this value.
Results.
| Patient | Age* | Length of time withouttreatment° | FENO 1 | Recurrence2 | Immunotherapy3 |
| 1 | 11 | 12 | 13 | Yes | Yes |
| 2 | 11 | 1 | 34 | Yes | Yes |
| 3 | 14 | 12 | 102 | No | Yes |
| 4 | 06 | 10 | 29 | No | Yes |
| 5 | 08 | 12 | 33 | No | Yes |
| 6 | 13 | 3 | 48 | No | Yes |
| 7 | 12 | 2 | 14 | Yes | No |
| 8 | 10 | 10 | 22 | No | Yes |
| 9 | 09 | 6 | 36 | No | Yes |
| 10 | 13 | 1 | 99 | No | Yes |
| 11 | 12 | 12 | 144 | No | Yes |
| 12 | 09 | 12 | 58 | No | Yes |
| 13 | 09 | 8 | 25 | Yes | Yes |
| 14 | 13 | 6 | 127 | No | Yes |
| 15 | 09 | 12 | 65 | No | Yes |
| 16 | 10 | 12 | 18 | No | Yes |
| 17 | 13 | 4 | 63 | No | Yes |
| 18 | 11 | 12 | 51 | No | Yes |
| 19 | 13 | 2 | 107 | No | Yes |
| 20 | 12 | 1 | 52 | No | Yes |
| 21 | 12 | 1 | 36 | No | Yes |
| 22 | 13 | 6 | 60 | No | Yes |
| 23 | 08 | 7 | 12 | No | Yes |
| 24 | 11 | 2 | 48 | No | Yes |
| 25 | 14 | 11 | 47 | No | Yes |
| 26 | 14 | 3 | 60 | Yes | Yes |
| 27 | 06 | 12 | 33 | Yes | Yes |
| 28 | 14 | 12 | 136 | No | No |
*Age in years. °Length of time without treatment prior to first visit, in months. 1FENO in ppb. 2Recurrence according to criteria, in the six months of monitoring. 3Immunotherapy: being in receipt, during the monitoring period, of treatment with allergen specific immunotherapy.
A correlation has been found in several studies between the value of the FENO and the level of inflammation of the airways.1–4 On the basis of this, attempts have been made to correlate FENO values with the level of bronchoconstriction16 and with the response to treatment with inhaled corticosteroids.17–19 FENO values are also a considerable aid in the diagnosis of allergen-induced asthma. The concordance of the values obtained using the portable devices with those obtained by the traditional devices13,14 has been checked, resulting in clear and universally applicable reference values. One study proposes that 49 ppb should be taken as the cut-off point, above which a significant probability of a further attack of asthma exists.15 However, there is currently some discussion about the usefulness of the FENO values as opposed to the traditional measurements in certain cases, such as that of clinically controlled patients who are not receiving baseline treatment.9–11 This raises the question of whether to treat the patient according to the clinical control and pulmonary function test or according to the data obtained in the FENO measurement, which may reveal a latent inflammation but one that we do not know to be sufficient to lead to recurrence. In our study we can see an even greater probability of losing control of asthma in the group with FENO values of lower than 49, with a low positive predictive value. In other words, asymptomatic and controlled patients who are receiving no treatment and with values of over 49 ppb have a low probability of suffering recurrence in the next six months, while in the group with values lower than 49 ppb more recurrence was observed. Although we acknowledge that the small sample size is a limitation in our study, it raises doubts about the use of high levels of FENO as the principal data on which to base the recommendation of baseline treatment in children with asthma which is under good control. These results should be considered and taken as a starting point of further studies with longer follow-up and a greater number of patients, in order to confirm them.
ConclusionsOn a first reading of the results obtained in our study it would appear unwise to recommend systematic treatment of patients with high levels of FENO obtained in a routine check-up in cases of allergy-based asthma which are currently asymptomatic or in a phase of good control. However, it will be necessary to broaden the range of this study, we recommend to carry out a more extended monitoring of the patients, as the predictive value beyond a six-month period of follow-up is not known, and also to increase the sample size before more specific recommendations can be made.
Conflict of interestThe authors declare that they have no conflict of interest.