Asthma starting in childhood can evolve in different ways1, though the persistence or reappearance of the disease in adulthood is dependent upon very diverse factors, some of which are well known, though others may go unnoticed due to difficulties in evaluating them or to special situations of concrete individuals, ethnic groups, climates or occupational environments, among others. The remission or relapse rates are highly variables in the different studies published, possibly because of differences in the populations studied, in the diagnostic criteria employed, or in the treatments prescribed2,3. Application of the term "natural history" in reference to the evolution of the disease is not fortunate, since it implies spontaneous evolution without therapeutic intervention4-6. Paradoxically, most follow-up studies make no reference to the treatment followed by the included patients, though it must be assumed that the medication and environmental measures adopted will have played a relevant role in the development of the process. The parameters most commonly addressed in such studies comprise patient sex, atopy and respiratory function, and some authors also contemplate bronchial lability7,8.
In most asthmatic children, atopic susceptibility is a key factor for development of the process, where an allergic etiology is accepted in up to 70 % of cases. Inflammation of the respiratory mucosa secondary to the allergic reaction, and bronchial hyperresponsiveness, constitute the two main pathogenic factors of the disease, which often begins manifesting locally in the form of rhinitis thus requiring the investigation of possible bronchial lability9,10.
The aim of the present study was to determine the opinion of patients regarding their asthmatic disease diagnosed in childhood, and that after several years without symptoms, had been discharged as cured. The initial severity at beginning the process and bronchial responsiveness at discharge were evaluated.
MATERIAL AND METHODS
A questionnaire was administered among patients diagnosed with extrinsic asthma and controlled for a number of years since childhood, with treatment according to the management options available in the years of disease control. The visits of the included patients had ceased after a minimum symptoms-free period of two years, without the need for medication, and all subjects were assessed for bronchial responsiveness based on the methacholine test performed at the time of medication suspension. Several years after the end of the controls, the patients were requested to answer the questionnaire to determine their health in relation to their previous asthmatic process (see Annex 1).
Patients
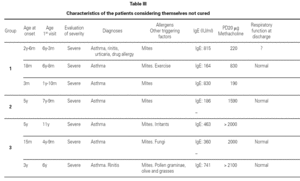
All the patients receiving the questionnaire met the following conditions: on the study made in the first visit in childhood, serum total IgE above the upper limit of normality for the age involved. Prick-test positive to one or more aeroallergens (predominance of dust house mites, followed by pollen of graminae, grasses, parietaria or olive, and Alternaria). In all patients, sensitization was confirmed via specific IgE testing (RAST).
The severity of the process was evaluated on the basis of the habitual need for treatment of the asthmatic crises, using the following criteria:
Mild: no medical consultation usually required (self-controlled), with no alteration of daily life activities.
Moderate: usually requires a medical visit, and alters daily life activities.
Severe: emergency care or hospital admission required on more than one occasion.
Very severe: admission to intensive care required on at least one occasion: status asmaticus.
Methacholine test
The abbreviated method of Yan was used, in which the aerosol is inhaled during inspiration, allowing quantification of the methacholine dose administered11. The patients were asymptomatic on performing the methacholine provocation test, without bronchodilating or antiinflammatory medication for at least the two preceding days, and with respiratory function parameters within the normal range, including FEV1 > 70 % the predicted value. Spirometry was carried out using the Vicatest Spimco (Mijnhardt, The Netherlands) before testing and two minutes after each of the inhalations (Mediprom FDC 88 dosimeter, Paris, France). With mouthpiece connection to the nebulizer (De Vilbiss 5610 D), the patients were allowed to breathe normally, and after forced exhalation were instructed to perform maximum inspiration (1-2 seconds), followed by a 3-second apneic period, and then gentle exhalation11,12. The drop in FEV1 was estimated from the value of this parameter recorded after inhalation of the saline solution with which the test is started. Dilutions of methacholine were prepared (Provocholine, Roche) 1/100 with saline solution, yielding concentrations of 10 mg/ml. In the first inhalation 100 μg of methacholine were administered, followed by repeated dosing of 200 μg (cumulative dosage: 300 μg, 500 μg, 700 μg, 900 μg, etc.). The test ended when FEV1 decreased 20 % (PD20), calculated from the dose-response curve. Administration was suspended if this decrease was not observed with a maximum cumulative dose of 2100 mg.
Statistical analysis
Fisher's exact test for small parametric samples was used, though some of the parameters with figures 5 were confirmed with the Chi Square Calculator.
RESULTS
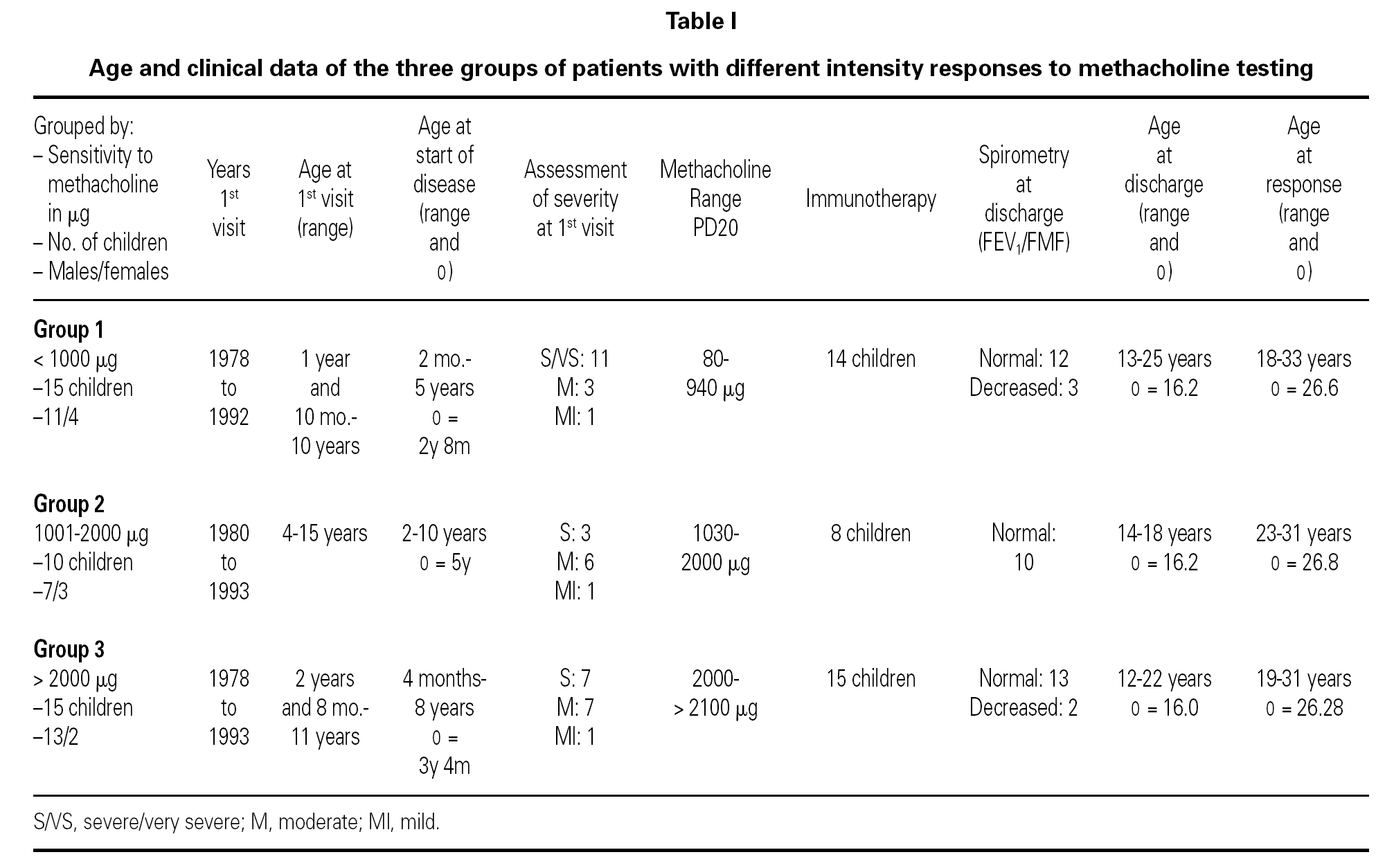
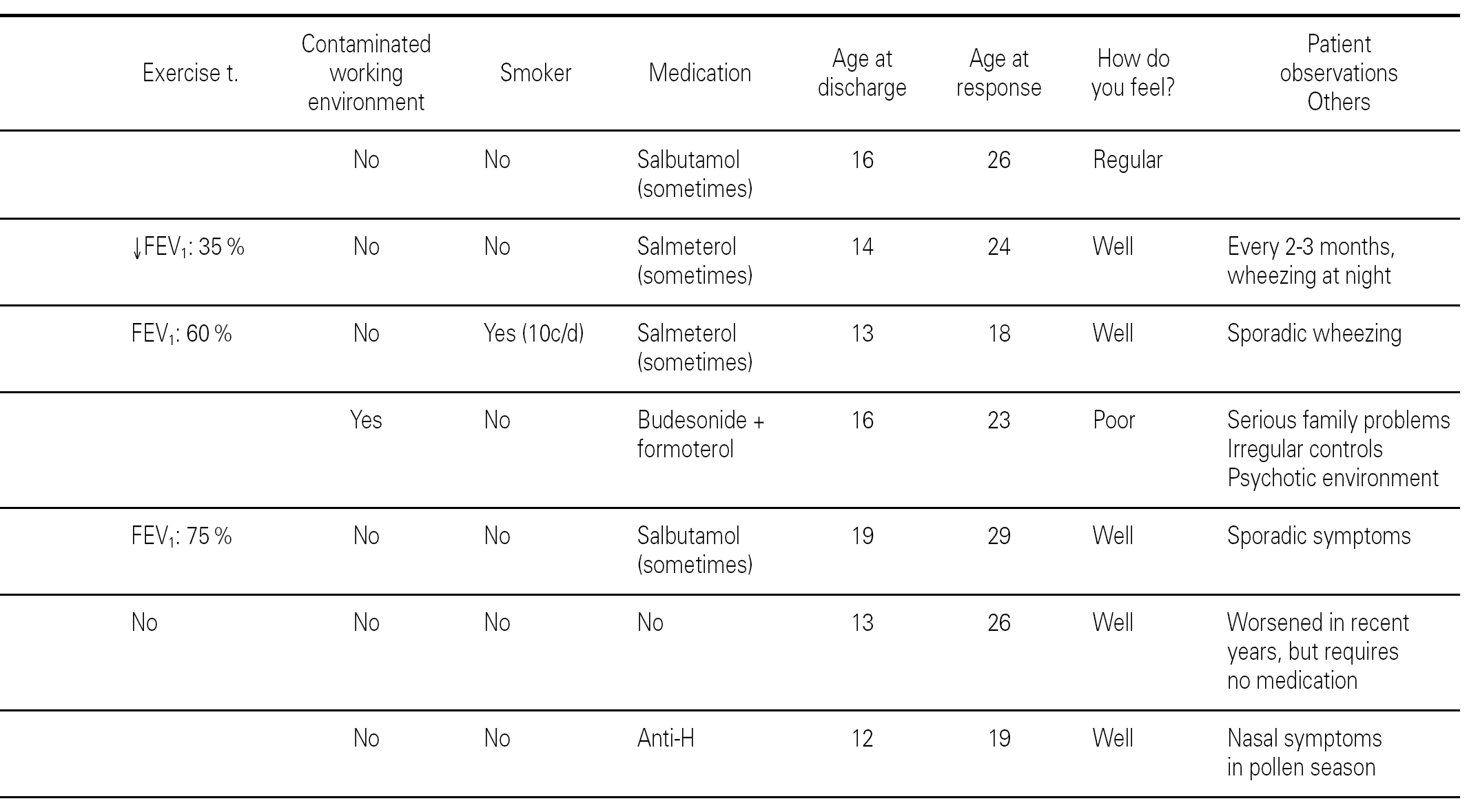
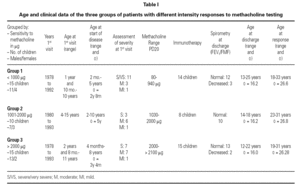
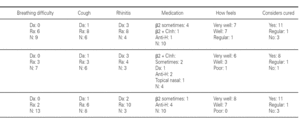
Of the 78 questionnaires delivered to the home addresses reflected in the case histories, 9 (11.5 %) were returned due to change in address, and 42 were answered (53.8 %). Of these, two were found to be incomplete and were excluded. The first visit of all the children took place between 1978 and 1993, and patient age on that first visit ranged from one year and 10 months to 15 years. The symptoms of asthma had started at ages between 2 months and 10 years, with an average of 3 years and 6 months (table I). The control of these patients ceased between the years 1991 and 2001.
Specific immunotherapy was the etiological treatment followed by 37 of the patients. Prophylactic, symptomatic or pathogenic treatment varied over the period of time in which the patients were controlled, depending on the habitual medication in each moment and the clinical condition of the patient. In all case, adoption of the pertinent environmental measures was recommended.
The global 40 children were divided into three groups according to the amount of methacholine required to reach PD20: group 1: < 1000 μg (very sensitive), 15 children; Group 2: 1001-2000 μg (moderately sensitive), 10 children; and Group 3: > 2000 μg (scantly sensitive), 15 children. Immunotherapy was prescribed in 14 children in group 1, 8 in group 2, and all 15 in group 3. Patient control continued up to an average age of 16.2 years (range 12-25), with a similar distribution in all three groups. At the end of the control period, respiratory function remained within normal limits (FEV1 ≥ 80 % and FMF25-75 ≥ 60 % of prescribed) in 78.5 % of the patients in group 1, in all the patients in group 2, and in 86.6 % of those in group 3.
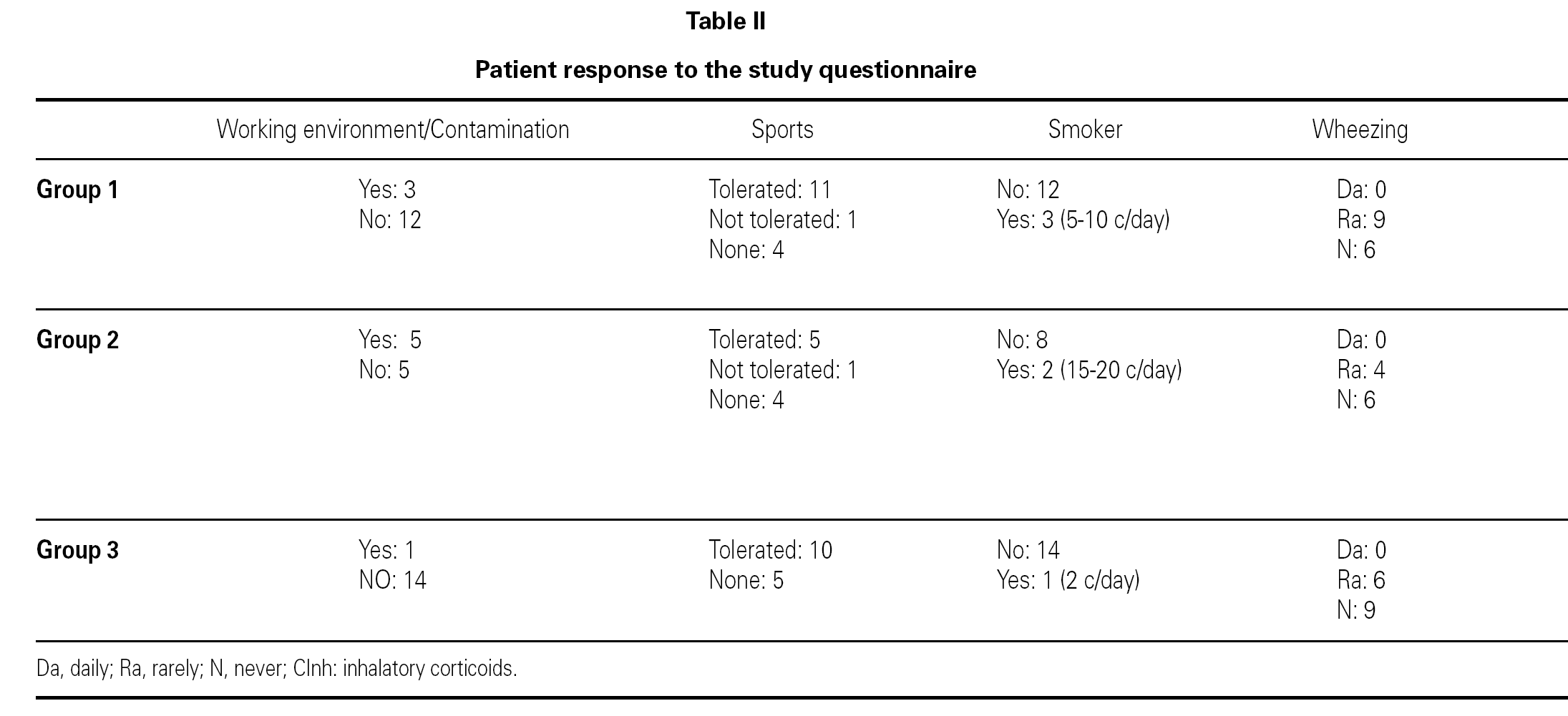
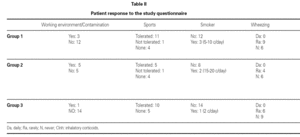
The ages of the patients at the end of the controls and on answering the questionnaire were similar in all three groups, with an average of 16 years (range 12-22) at control cessation, and 26 years (range 18-31) at the time of the questionnaire. In this context, the responses were as follows (table II):
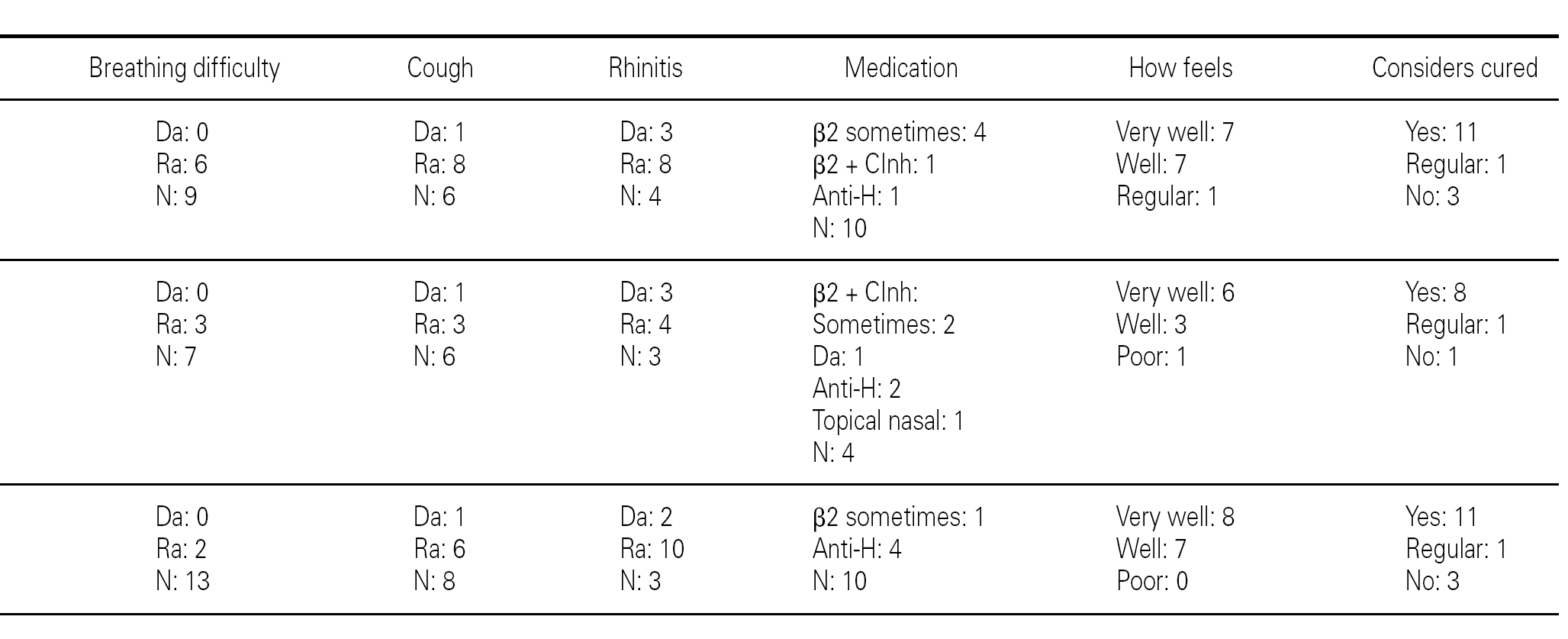
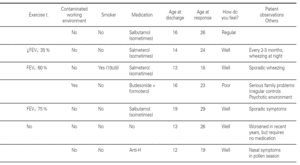
Group 1 (very sensitive): Three works in a contaminated environment, and three are moderate smokers (< 10 cigarettes/day); 11 tolerate sports activities well, one does not tolerate such activity, and four do not participate in such activities. Sporadically, 9 suffer wheezing, 6 breathing difficulty, 8 cough, and 8 symptoms of rhinitis. On a daily basis, one suffers cough and three rhinitis. The use of medication is very limited, since 10 of the patients usually never require pharmacological treatment. Seven claim to feel very well, another 7 well, and one regular. In sum, 11 consider themselves to be cured, 3 do not consider themselves to be cured, and one patient describes personal condition as "regular".
Group 2 (moderately sensitive): Half of the subjects work in a contaminated environment, and two are smokers (15-20 cigarettes/day). Five tolerate sports activities, one does not, and four do not participate in such activities. Sporadically, four suffer wheezing, three breathing difficulty and cough, and four have symptoms of rhinitis. On a daily basis, only one patient suffers cough, and three rhinitis. Four patients never use medication, one uses topical antihistamines, and two sometimes a β-agonist and/or inhaled corticoids. Six feel very well, three well, and one poorly. Eight patients consider themselves to be cured, one does not, and one patient describes personal condition as "regular".
Group 3 (scantly sensitive): Only one of these patients works in a contaminated environment, and one smokes no more than two cigarettes a day. Ten tolerate sports activities, while the remaining 5 do not practice sports. Sporadically, 6 suffer wheezing, two breathing difficulty, 6 cough and 10 rhinitis. On a daily basis, only one patient coughs, and two present symptoms of rhinitis. Four sometimes use antihistamines, and one patient uses β2-agonists. Eight claim to feel very well and 7 well. Eleven patients consider themselves to be cured, three do not, and one patient describes personal condition as "regular".
No significant differences were recorded on comparing the results of the three groups in terms of the most characteristic symptoms of bronchial involvement (wheezing and breathing difficulty), and as refers to the opinion of the patients on their personal condition and whether or not they consider themselves to be cured.
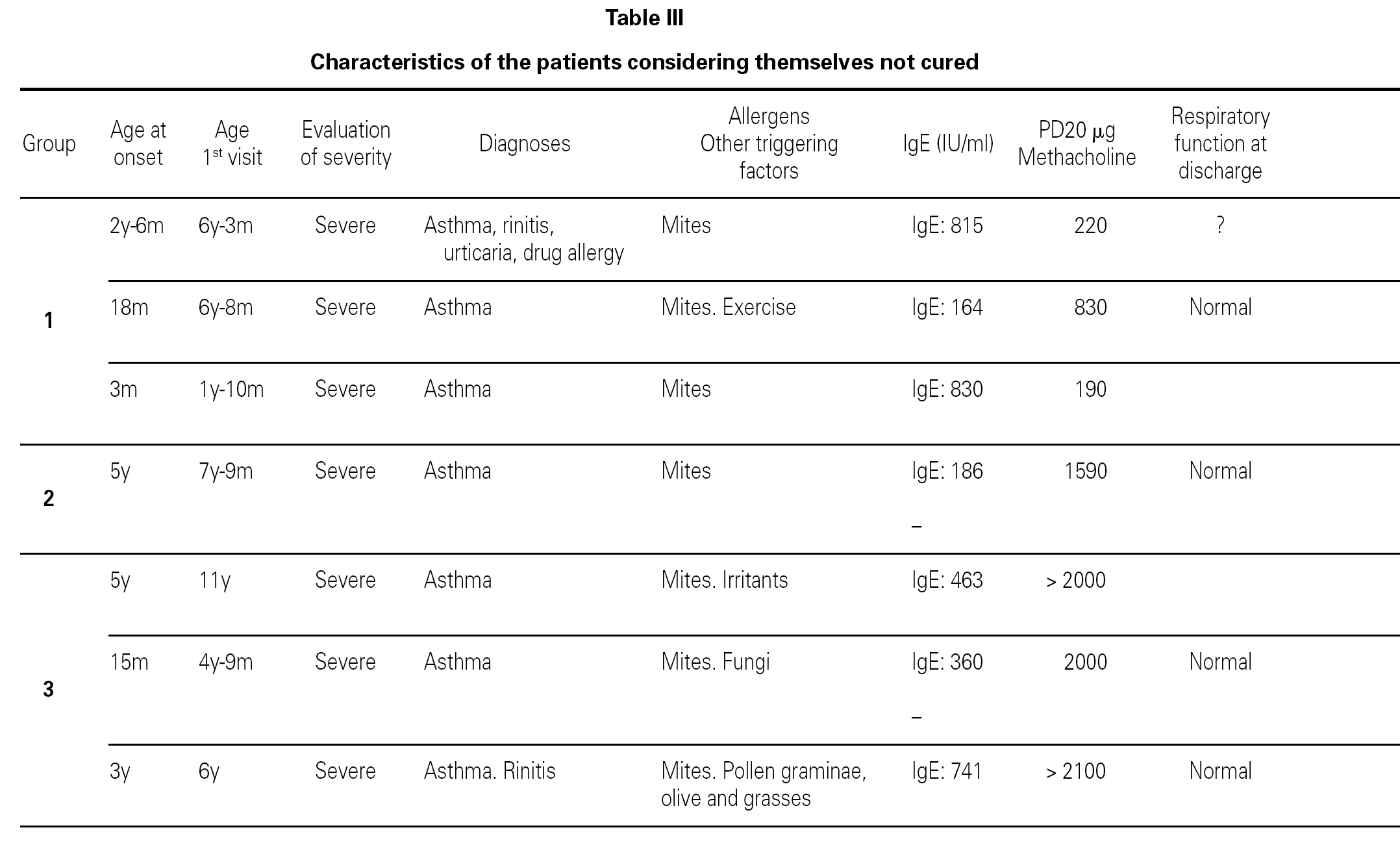
Most of the patients in all three groups considered themselves to be cured (11, 8 and 11 subjects, respectively). One in each group considers healing to be incomplete ("regular"), due to the existence of sporadic symptoms. Lastly, three patients in group 1, one in group 2, and three in group 3 do not consider themselves to be cured. The characteristics of these patients are shown in table III.
DISCUSSION
A range of factors are implicated in the appearance and progression of asthma, and their role is difficult to establish in each concrete case, due to the influence of genetic predisposition (atopy), patient life style habits and the characteristics of the environment (in the home, outdoors and at work). The age at onset of the disease, the persistence and severity of the symptoms, the presence of eczema as associated topical pathology, the timeliness and idoneity of treatment and its correct compliance, and patient gender (increased frequency among males in infancy, with female predominance at later ages), are all aspects to be taken into consideration in prognosing possible persistence or reappearance of the disease in the adult2,3,7,13,14. Of all the underlying factors, special attention should focus on patient respiratory status both static (spirometry) and functional (bronchial hyperresponsiveness)7,13,15.
Bronchial hyperresponsiveness is known to play a fundamental role in the pathogenesis of asthma, and it is difficult to establish a diagnosis in its absence16,17.
The method most commonly used to evaluate the degree of bronchial hyperresponsiveness is based on the inhalation of methacholine at progressively increasing doses. A number of procedures have been developed to this effect, of which two are currently recommended: the Two-minute tidal breathing dosing protocol and the Five-breath dosimeter protocol18. The effectiveness of the two protocols does not differ from that of other abbreviated methods, which are easy to perform and offer the advantage of providing a more accuracy of the methacholine dose administered11,15,19-21.
The patients of this study were grouped according to their response to methacholine provocation as very sensitive (PD20 with < 1000 μg), moderately sensitive (PD20 between 1000 and 2000 μg), and scantly sensitive (PD20 > 2000 μg). Apart from immunotherapy, which was the treatment common to almost all children, baseline therapy was provided according to the management guidelines applicable in the years when the patients were subjected to control with variations in each case over time according to the clinical circumstances of each patient22.
Despite the different responses to methacholine provocation or challenge in the three groups, the evolution of the patients between 6 and 15 years (mean = 10.4) after the cessation of routine control did not differ to any important degree. The only numerical difference of note was sporadically perceived breathing difficulty, which proved to be more frequent among the children in group 1 (most sensitive) than in group 3 (least sensitive) though the difference was not statistically significant. The number of patients conforming the groups was possibly insufficient to demonstrate significant differences in the data collected by the questionnaire.
Since rhinitis is an associated process in practically all patients with asthma, and moreover considering that it is not always easy for patients to relate symptoms of rhinitis with the cause of allergy (most reporting rhinitis with less or greater frequency), we have avoided analyzing the responses to this item of the questionnaire.
Estimation of the severity of asthma has been the subject of a number of classifications, based on the frequency of the symptoms (seasonal incidence not being taken into consideration), the intensity of the asthmatic crises, or on patient respiratory function though none of these evaluations are full satisfactory23,24. The frequency and intensity of the asthmatic crises may be valid as a parameter for assessing severity, as well as the need or not for medical consultation or hospital admission, this being the parameter used in our patients on occasion of the first visit22. Despite the fact that the percentage of patients classified as severe or very severe in group 1 was considerably greater than in group 3 (73.3 % vs. 46.6 %), the difference failed to reach statistical significance possibly due to the reduced sample size involved.
Based on this classification, and on occasion of the first visit, 20 patients were considered to present severe asthma (plus another case considered to be very severe). Of these, 7 did not consider themselves to be cured, though contradictorily five claimed to feel well, and almost all defined the symptoms as sporadic (table III).
The future of patients who have suffered asthma since childhood should be a matter of concern. The evolution of the disease may be highly variable, though it is largely dependent upon the timeliness and idoneity of treatment. In the patients of our study, the time lag between the onset of the disease and patient age at the first visit is due to the fact that most of the subjects were treated by their pediatrician or by other specialists with no specification of the treatments prescribed. Methacholine testing was carried out at discharge after at least two years without symptoms and without treatment, in order to obtain an objective basis for establishing a long-term prognosis, due to the possibility of relapse with rates that differ greatly in the different studies found in the literature. In all cases, measures were advised to avoid long-term relapse, stressing the need to avoid smoking and working in contaminated environments (irritants, allergens). This could be referred to as "fourth prevention", as a complement to the preventive methods advocated in earlier ages and which are defined as "primary, secondary and tertiary prevention"25.
The three groups of patients were established according to the degree of hyperresponsiveness, which appeared to be the most objective parameter since other data may be difficult to evaluate, such as family antecedents, the association of other allergic processes, patient sex, exposure to aeroallergens, environmental contaminants (external and occupational), or smoking, etc. Other studies, some even published by the same authors3,26, differ in their appraisal of the predictive value of bronchial responsiveness in relation to the future of the patients14,27. Given the evolution of our patients, it can be deduced that the degree of bronchial responsiveness is not adequate as a parameter on which to base the middle-term prognosis of the risk of asthma reappearance in patients with processes that started in early childhood.
Different studies show that bronchial responsiveness improves with specific immunotherapy, with correction of much of the imbalance between Th1/Th2 lymphocytes this ratio being typically altered in atopic subjects28,29. In this sense, the patients in our third group possibly could have benefited from immunotherapy, since they required over 2000 mg of methacholine to reach PD20, and some even failed to reach this point with the maximum administered dose despite the fact that in 7 of them asthma was initially classified as severe, and moderate in another 7.
In conclusion, no correlation was found between the degree of bronchial hyper-responsiveness and the risk of relapse in young adults who suffered asthma in childhood. We consider that the favorable evolution of the patients is largely attributable to the specific treatment prescribed as soon as the etiological diagnosis of the process is established, together with the introduction of other measures that always should be decided as soon as possible30-32.
ACKNOWLEDGEMENTS
The author thanks Dr. Martin Rios-Alcolea, head of the Department of Statistics of the Faculty of Biology (University of Barcelona) for his valuable contribution to this study.
Correspondence:
Dr. F. Muñoz-López
Fax (34) 932 118 248
E-mail: 5314fml@comb.es