The aim of this study was to systematically review the safety and efficacy of inhaled beclomethasone for asthma treatment in pregnant women. We performed a systematic review in Medline, LILACS and SciELO electronic databases in December 2012. A total of 3433 articles were found by using the keywords asthma, pregnancy and beclomethasone. Among these, 1666 were from Medline, via PubMed, and 1767 were from LILACS and SciELO. Nine of these articles were selected. Only one paper suggested an increased foetal risk for congenital malformations, and one other for offspring endocrine and metabolic disturbances. Data are mostly reassuring, supporting the use of glucocorticoid inhalants during pregnancy, and we found no evidence of inferiority in relation to efficacy and safety of beclomethasone compared to other drugs used in pregnant asthmatic women.
Asthma is a chronic disease of lower airways, characterised by inflammation and increased responsiveness, which results in bronchial obstruction, partially or completely reversible and symptoms of dyspnoea, wheezing, cough and expectoration, which vary in intensity and frequency from patient to patient and in the same patient at different times.1 Acute exacerbations can mainly occur with triggers by exposure and viral infections of airways.1 It is the most common chronic disease among women in reproductive age2 and can affect up to 8% of pregnant women.3 When inadequately controlled, it is associated with increased risk for prematurity, low birth weight, congenital malformations, preeclampsia and caesarean section.4
Adverse outcomes of asthma can be avoided with proper control through educational, behavioural and pharmacological actions,1,5 especially with the use of inhaled corticosteroids, whose efficacy and safety in pregnant asthmatic women have been extensively studied in recent years.6,7
One of the largest studies was the Swedish cohort published by Norjavaara and De Verdier (2003), which analysed the safety of budesonide in 2968 pregnant women.8 Since then, this inhaled corticosteroid has been considered the most studied and safe for use in pregnant women and considered as class B by the American organ of Food and Drug Administration (FDA). There are no comparative studies about asthma treatment during pregnancy between beclomethasone and budesonide,7 so, beclomethasone is being considered class C because of the limited available studies and not due to the presence of a known risk.
The knowledge of available evidence about the safety and efficacy of inhaled beclomethasone is important for clinical practice and the implementation of public health actions in the treatment of asthma because it is an accessible drug, with a low cost and, in Brazil, it is distributed for free by the public health service. The objective of this study is to present a systematic review about the efficacy and safety of beclomethasone used to treat pregnant asthmatic women.
Search strategyThe systematic literature review was performed through Medical Literature Analysis and Retrieval System Online (MEDLINE), Latin American and Caribbean Health Sciences (LILACS) and Scientific Electronic Library Online (SciELO) databases and the search data occurred in December 2012. A specific strategy was elaborated for each of them to the intersection of the descriptors (DECS) – keywords for retrieving subjects from scientific literature.
For Medline/PubMed, the search strategy was performed in two stages using the syntaxes (“Asthma”[Mesh]) AND “Pregnancy”[Mesh] and (“Beclomethasone”[Mesh]) AND “Pregnancy”[Mesh]. For LiLACS and SciELO, the keywords “asthma”, “beclomethasone” and “pregnancy” were used.
Selection criteriaTo be included in the analysis studies should be original articles (excluding editorials and review studies); have pregnant asthmatic women submitted to treatment with inhaled corticosteroids including beclomethasone, as research subjects; approach some of the following outcomes: congenital malformations risk, premature birth, caesarean delivery, intrauterine growth restriction, asthma exacerbations, pre-eclampsia, or childhood diseases; and be published in Portuguese, English or Spanish. Studies that did not define the number of patients using beclomethasone were excluded.
Data analysisThe articles selection consisted of three steps. The first one was based on reading the title of the articles. Those that clearly did not fit in any of the criteria for inclusion in this study were excluded. In the second step, the abstract of each selected article was read and, likewise, those that did not fit in any of the inclusion criteria were rejected. In the third step, the articles that were not excluded were fully read in the aim of selecting those which would be included in this review.
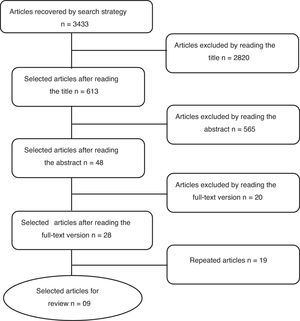
Crossing the keywords “asthma” and “pregnancy”, and “beclomethasone” and “pregnancy” in the Medline database, via PubMed, 1666 articles were found. Among these, 1347 articles were eliminated due to the title, 292 were excluded by reading the abstracts and 12 after reading the full-text version. Nine non-repeated studies were selected for review. In the SciELO database, crossing the keywords “asthma” and “pregnancy”, 13 articles were found and all of them were eliminated by the title. In the LILACS database, crossing the same keywords as for PubMed, 1717 articles were retrieved, 1430 were excluded by the title, 273 after abstract and 8 after full text reading. As a result, six studies were selected, all of them already selected, thus leaving the same nine articles retrieved through the PubMed search (flowchart shown in Fig. 1).
ResultsThe heterogeneity of articles did not allow to group them for a statistical analysis, so sufficient data was not found to conduct a meta-analysis. Because of this, the results of this study will be presented as a systematic review. According to the “Cochrane Library”, if it is not possible to do a meta-analysis, the researcher should feel encouraged, by the line of research, on building a field for randomised clinical trials.9
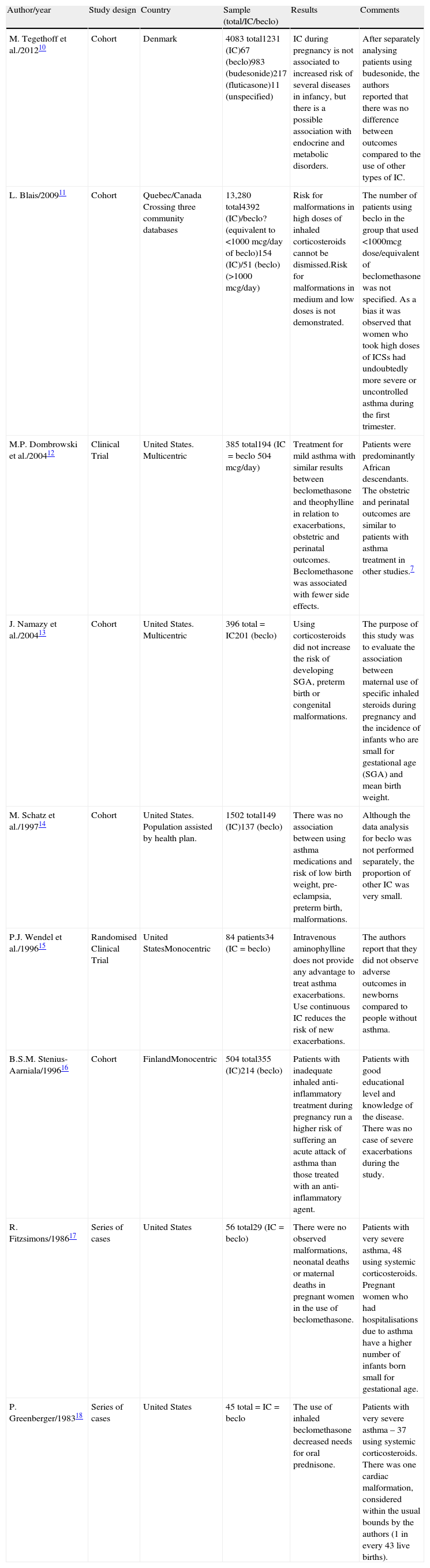
It was decided to consider the following variables of the selected articles: author/year, study design, country, sample (total, using inhaled corticosteroids and using beclomethasone) and results, with the aim of doing a better presentation (Table 1).
Selected studies about using beclomethasone in pregnancy.
| Author/year | Study design | Country | Sample (total/IC/beclo) | Results | Comments |
| M. Tegethoff et al./201210 | Cohort | Denmark | 4083 total1231 (IC)67 (beclo)983 (budesonide)217 (fluticasone)11 (unspecified) | IC during pregnancy is not associated to increased risk of several diseases in infancy, but there is a possible association with endocrine and metabolic disorders. | After separately analysing patients using budesonide, the authors reported that there was no difference between outcomes compared to the use of other types of IC. |
| L. Blais/200911 | Cohort | Quebec/Canada Crossing three community databases | 13,280 total4392 (IC)/beclo? (equivalent to <1000mcg/day of beclo)154 (IC)/51 (beclo) (>1000mcg/day) | Risk for malformations in high doses of inhaled corticosteroids cannot be dismissed.Risk for malformations in medium and low doses is not demonstrated. | The number of patients using beclo in the group that used <1000mcg dose/equivalent of beclomethasone was not specified. As a bias it was observed that women who took high doses of ICSs had undoubtedly more severe or uncontrolled asthma during the first trimester. |
| M.P. Dombrowski et al./200412 | Clinical Trial | United States. Multicentric | 385 total194 (IC=beclo 504mcg/day) | Treatment for mild asthma with similar results between beclomethasone and theophylline in relation to exacerbations, obstetric and perinatal outcomes. Beclomethasone was associated with fewer side effects. | Patients were predominantly African descendants. The obstetric and perinatal outcomes are similar to patients with asthma treatment in other studies.7 |
| J. Namazy et al./200413 | Cohort | United States. Multicentric | 396 total=IC201 (beclo) | Using corticosteroids did not increase the risk of developing SGA, preterm birth or congenital malformations. | The purpose of this study was to evaluate the association between maternal use of specific inhaled steroids during pregnancy and the incidence of infants who are small for gestational age (SGA) and mean birth weight. |
| M. Schatz et al./199714 | Cohort | United States. Population assisted by health plan. | 1502 total149 (IC)137 (beclo) | There was no association between using asthma medications and risk of low birth weight, pre-eclampsia, preterm birth, malformations. | Although the data analysis for beclo was not performed separately, the proportion of other IC was very small. |
| P.J. Wendel et al./199615 | Randomised Clinical Trial | United StatesMonocentric | 84 patients34 (IC=beclo) | Intravenous aminophylline does not provide any advantage to treat asthma exacerbations. Use continuous IC reduces the risk of new exacerbations. | The authors report that they did not observe adverse outcomes in newborns compared to people without asthma. |
| B.S.M. Stenius-Aarniala/199616 | Cohort | FinlandMonocentric | 504 total355 (IC)214 (beclo) | Patients with inadequate inhaled anti-inflammatory treatment during pregnancy run a higher risk of suffering an acute attack of asthma than those treated with an anti-inflammatory agent. | Patients with good educational level and knowledge of the disease. There was no case of severe exacerbations during the study. |
| R. Fitzsimons/198617 | Series of cases | United States | 56 total29 (IC=beclo) | There were no observed malformations, neonatal deaths or maternal deaths in pregnant women in the use of beclomethasone. | Patients with very severe asthma, 48 using systemic corticosteroids. Pregnant women who had hospitalisations due to asthma have a higher number of infants born small for gestational age. |
| P. Greenberger/198318 | Series of cases | United States | 45 total=IC=beclo | The use of inhaled beclomethasone decreased needs for oral prednisone. | Patients with very severe asthma – 37 using systemic corticosteroids. There was one cardiac malformation, considered within the usual bounds by the authors (1 in every 43 live births). |
IC: inhaled corticosteroids; beclo: beclomethasone; total: number of patients in the study; SGA: small for gestational age.
The first aspect to emphasise, after analysing the articles, is the lack of studies designed to specifically evaluate beclomethasone: only two out of nine articles.12,18 Because of ethical reasons, the difficulty of proposing studies to evaluate drugs in pregnant women is understood. However, the subject deserves more attention because beclomethasone has been largely used in the past thirty years to treat pregnant asthmatic women. In fact, beclomethasone continues to be recommended as an alternative for budesonide during pregnancy by internationals protocols.19 Most articles discuss about the efficacy and safety of inhaled corticosteroids in general, which is a recurrent subject of research in this area.11,13–17
The first publications from the 1980s sought to evaluate the drug efficacy and safety in a period in which it had never been used in pregnant women.17,18 As a result, several case series studies emerged, which were important to suggest safety of using corticosteroids and to stimulate the emergence of new studies in the 1990s, at this time, more capable to evaluate risks and efficacy.14–16 Cohort studies and clinical trials which were produced in the 1990s came to consolidate the idea of safety and efficacy of inhaled corticosteroids used in pregnant asthmatic women. In these studies, beclomethasone was the most used inhaled corticosteroid.
Publications about asthma treated in pregnancy with others drugs have become rare after the publication of a cohort study that evaluated budesonide in pregnant women in 2003.8 Four articles10–13 published after 2003 were found in this review. Among these, two prospective studies had their data collection started before 2003 and discussed about the use of beclomethasone or inhaled corticosteroids, which more than a half of the sample used beclomethasone.12,13 The only two articles actually designed after the publication of 2003 and selected for this review are cohort studies taken from Canadian and Danish databases, with pregnant asthmatic women evaluated between 1990–200211 and 1996–2002.10 In the Canadian study, beclomethasone was, once again, one of the most used inhaled corticosteroids; however, the study of Denmark used beclomethasone in a small number of patients (5.4%).
Although asthma is a disease present around the world, the articles analysis demonstrates a regionalisation of publications, in which all searches were conducted in developed countries. Among the nine selected articles, six are from the United States, one is from Canada, one from Finland and one from Denmark. There are no articles from Central America, South America, Africa or Asia and only three of them were made outside of America. There is a lack of scientific studies conducted in countries with less favourable socio-economic conditions, where more interest in verifying the efficacy and safety of a low cost drug to use in pregnancy is to be expected.
The sample size was also an important aspect in the analysis of articles. A lack of data about beclomethasone was noticed. We found 960 women using beclomethasone in all the selected articles, while just a single cohort study that evaluated budesonide has got 2968 pregnant women.8 In addition, several articles aimed to verify the effects of inhaled corticosteroids, but they might be questionable for extrapolating the results to beclomethasone.
The first studies from the United States suggested that beclomethasone, when used in pregnant women, is a viable alternative and it declines the necessity for oral corticosteroid.17,18 According to a study published in Finland, inhaled corticosteroids reduced the risk of acute asthma exacerbations during pregnancy.16 Wendel et al. published a clinical trial which showed no advantage in the use of aminophylline and also found a decreased risk of new asthma exacerbations among patients using inhaled corticosteroids.15
The author who was more frequently found in the selected articles was M. Schatz. He participated in all the studies from the United States since 1997. The first of them was a cohort study with 1542 pregnant asthmatic women, 149 of whom were using inhaled corticosteroids and, among these, 137 were using beclomethasone.14 No association was found between using corticosteroids and increased risk of poor outcomes like low birth weight, pre-eclampsia, prematurity and malformations. Seven years later, two more studies were published: a cohort study that found no association between using inhaled corticosteroid and risk of intrauterine growth restriction,13 and a well-designed clinical trial, which aimed to study specifically beclomethasone. It was a randomised double-blind study with a sample of 385 patients. In this study, theophylline was compared to beclomethasone12 and the results showed that beclomethasone has a similar efficacy and safety as theophylline, although with less side effects.
The only study that reported poor perinatal outcomes in women who used inhaled corticosteroids during pregnancy was a Canadian study, which was taken from a cohort study found in a database from Quebec.11 Increased risk for congenital malformations among patients using high doses of the drug was reported by this study. However, patients who used high doses of corticosteroids also had more severe and uncontrolled asthma, so it is not possible to infer causality or safety due to a selection bias.
The most recent article selected by this review is a Danish cohort study, which evaluated 4083 asthmatic patients.10 This is the only article which relates inhaled corticosteroids in pregnancy and development of diseases in childhood. In this study, beclomethasone corresponds to only 5.4% of patients, while budesonide was used by 79.9%. There was no increased risk for several diseases in childhood, but it has a possible association with endocrine and metabolic disorders. The authors compared patients who used only budesonide in pregnancy with those who used other inhaled corticosteroids, but they did not find any differences.
ConclusionIn this review, most of the studies attest safety and efficacy of using inhaled corticosteroids in pregnant asthmatic women. Considering the articles selected by search strategy, beclomethasone was the most commonly used drug and it was separately evaluated in two articles. We did not find evidence of inferiority or lesser safety of using beclomethasone compared to other drugs indicated for asthma management during pregnancy, in the selected articles.
A greater concern is noticed regarding the safety of using inhaled corticosteroids instead of a specific corticosteroid, such as beclomethasone. The application of beclomethasone in scientific articles, which evaluate asthma during pregnancy, is absent after the Norjavaara publication and classification of budesonide as class B by FDA. The evidence of budesonide safety, reported by a population-based cohort study in which no other drug was evaluated, made the production of new articles about other inhaled corticosteroids ethically difficult.
In conclusion, the knowledge about the use of beclomethasone does not show evidence of a high risk for treatment of pregnant asthmatic women. However, budesonide has been better studied in literature and because of that it is considered the first choice in the management of asthma during pregnancy. In this context, it is difficult to imagine new studies that explore the use of beclomethasone in pregnant women. It is interesting to conduct observational studies in countries where beclomethasone is used due to more affordable costs compared to budesonide.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.