INTRODUCTION
In the field of allergic diseases, fungal sensitization is one of the least known and investigated aspects, due to the difficulties involved in conducting aerobiological studies to define the geographic and seasonal distribution of the spores of the main fungal species transported by air, and in establishing their concentration in both the external environment and in the home.
The prevalence of fungal allergies is greater than previously believed; consequently, such processes have been underestimated as potential causes of bronchial asthma and other respiratory tract diseases. In effect, the data on the prevalence of these disorders vary greatly. In 1995, a study was carried out sponsored by the Sociedad Española de Alergología e Inmunología Clínica1 in which 9.5 % of all patients with suspected respiratory allergy were seen to be sensitized to Alternaria and/or Cladosporium. According to a European multicenter study sponsored by the Aerobiology Subcommittee of the European Academy of Allergology in 1997, the prevalence of fungal sensitization in Spain was 20 %2. In the Alergológica 2006 study, in its pediatric section, 12.5 % of the patients with rhinitis and 15 % of those diagnosed with asthma were sensitized to fungi3. In the Escape study4, fungal sensitization in children was seen to reach 17.7 %.
1 Presented in Round Table at the XXXI SEICAP Congress, Córdoba (Spain), may 2007.
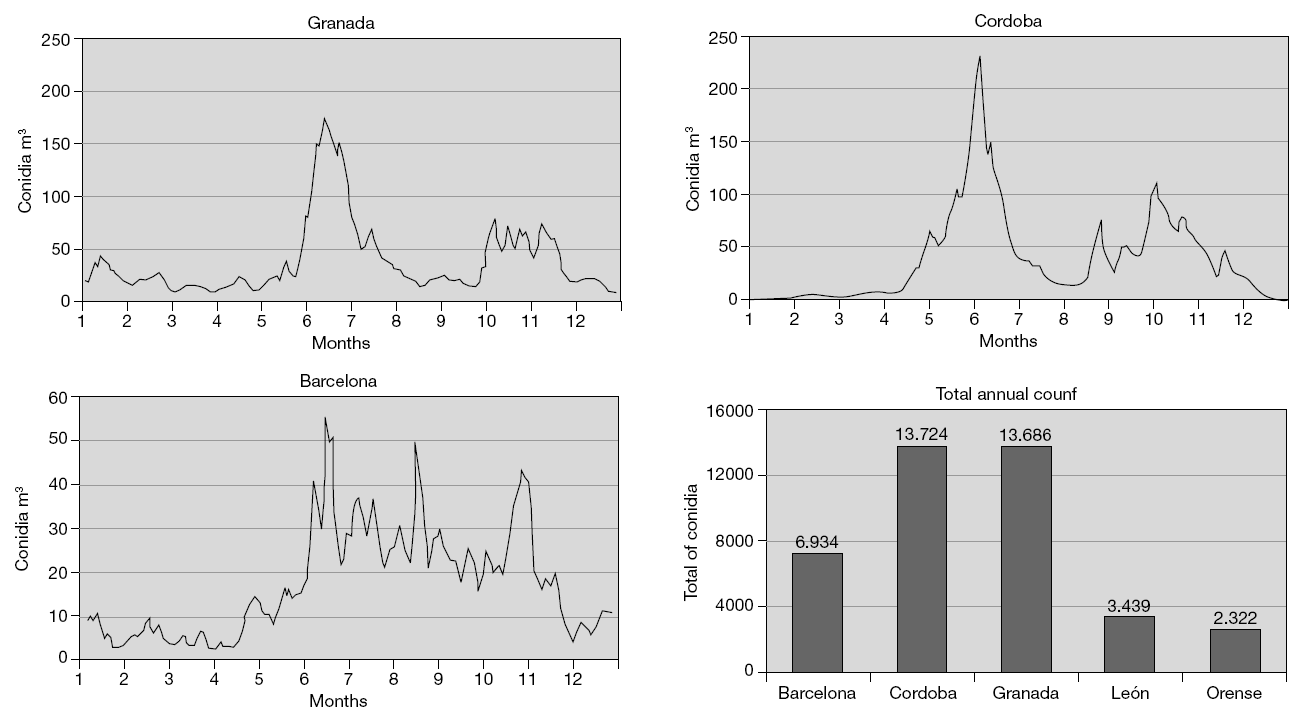
Most patients with fungal allergy have perennial symptoms5, though in our geographical setting the maximum concentration of Alternaria spores in the environment, and thus in theory the peak in symptoms among affected patients, corresponds to the summer and autumn months6 (fig. 1). These two peaks vary from one zone to another, as a result of the important relationship between meteorological parameters and spore concentrations. In effect, increases in temperature, humidity, cumulative rainfall increments, and the hours of sunlight all positively influence the levels of conidia detected in the air (particularly as refers to Alternaria).
Figure 1.--Levels of Alternaria conidia in different Spanish cities. Taken from: Infante F, Alba F, Caño M, Castro A, Domínguez E, Méndez J, Vega A. A comparative study of the incidence of Alternaria conidia in the atmosphere of five Spanish cities. Polen 1999; 10: 5-13.
Skin reactions to the antigens of Alternaria alternata are associated with a high risk of allergic respiratory conditions in the presence of spores of this fungus fundamentally in children and young adults7 with a special form of presentation as life-threatening asthma8. Severe wheezing is much less frequent in these two age segments, with an incidence of about 2 %9,10. These data must be taken into account when defining preventive, therapeutic and patient education measures11. Considering that allergic disease manifests gradually, with rhinitis and conjunctivitis preceding asthma, a number of authors have suggested that immunotherapy should be introduced in the first stages of allergic pathology12, in order to modify the natural course of the latter, before posterior sensitizations occurs, and to prevent the destruction of asthma remodeling observed over the long term. In addition, it has been seen that long-lasting, very severe or non-reversible allergic diseases respond poorly to immunotherapy.
Specific immunotherapy (IT) with allergens would thus act in the three phases of prevention:13
I.Primary prevention: avoiding further sensitizations.
II.Secondary prevention: altering the natural course of the respiratory allergy.
III.Tertiary prevention: ameliorating already established disease.
Very few controlled studies have examined the efficacy and safety of fungal extract immunotherapy14-19. As a result, the Immunotherapy Subcommittee of the European Academy of Allergology and Clinical Immunology has pointed to the need for controlled studies involving immunotherapy with standardized fungal extracts20.
The success of IT depends on the use of high-quality and adequately standardized allergenic vaccines that can be manufactured in a homogeneous manner21. It is necessary to add quantifications of predominant allergens to the standardization requirements, in order to develop adequate dosing regimens, and to determine the dose-response relationship between the predominant or majority allergens and the therapeutic effect elicited. It has been shown that measurement of the predominant allergens is correlated to the estimation of biological potency20.
One of the main problems for the study, diagnosis and treatment of fungal allergies is standardization of the fungal allergens, due to the variability of such extracts in terms of their potency and allergenic content. In recent years, Alternaria alternata and Aspergillus fumigatus have been the most extensively studied fungal species. Biological standardization has been achieved in these cases22, and recombinant extracts are used in clinical practice.
Since 1988, standardization studies have been published in relation to Alternaria, suited to the treatment and diagnosis of patients with allergy to this fungus23-26. Few studies have been conducted on the application of IT involving an Alternaria extract, and even fewer surveys have focused on children only. Cantani et al.27, in a prospective case-control study of IT with Alternaria (80,000 PNU), involving 39 children with rhinitis and/or asthma due to Alternaria sensitization, recorded no systemic reactions. In effect, only mild local reactions were seen, with evident clinical improvement among the patients in the active treatment group. Tabar et al.19 in turn analyzed a group of 46 children (aged 5-14 years), among the 129 patients comprising the study. These were patients with asthma and/or rhinitis secondary to Alternaria allergy, subjected to IT (5 BU/ml). In this age group there were more systemic reactions with the treatment starting dose, though not with the maintenance doses a significant difference being observed in this sense with respect to the adults. The immunotherapy group of the SEICAP conducted a multicenter tolerance study in pediatric patients28, in which good tolerance of the extract was observed with a low percentage of adverse reactions (0.95 % incidence of both local and systemic reactions at the dose administered).
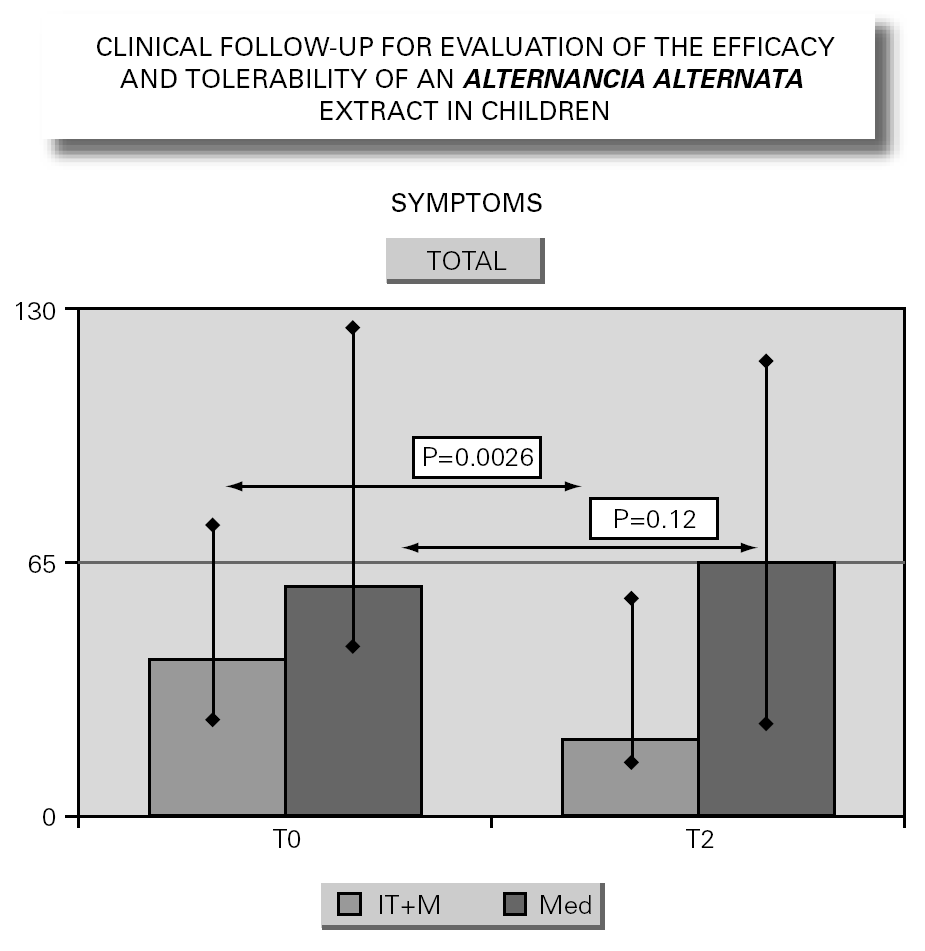
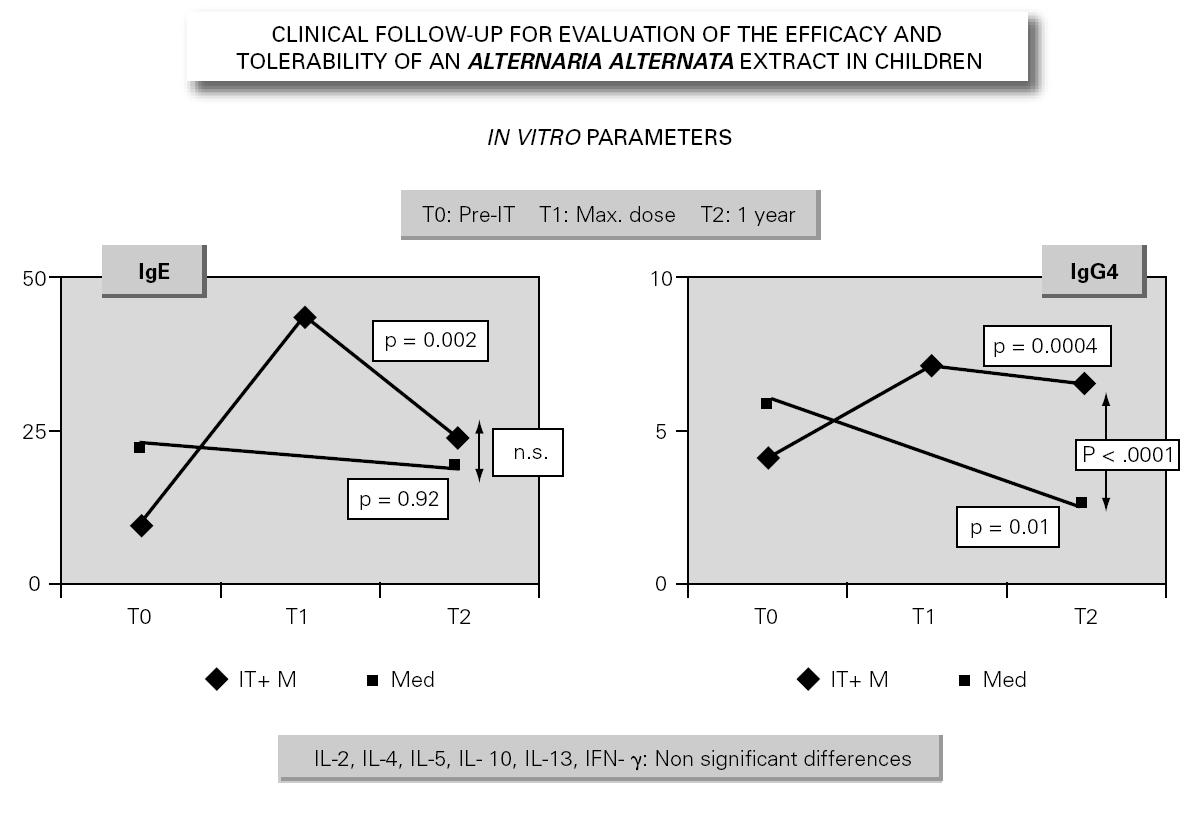
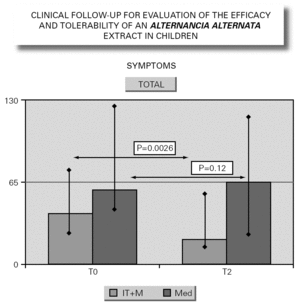
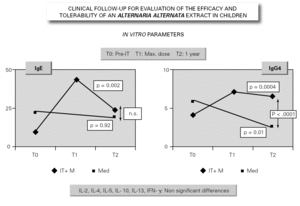
In parallel to this study, a clinical survey has been carried out among pediatric patients sensitized to Alternaria that reported to Virgen de las Nieves Hospital (Granada, Spain) on occasion of a first visit. After confirming the diagnosis of allergic respiratory pathology due to Alternaria sensitization, the patients were invited to participate in a special one-year follow-up evaluation. The children were randomized to either an active group (22 patients) administered Alternaria IT (Pangramin® Depot-UM, Alternaria alternata 100 %; ALK-Abelló, S.A.) in cluster regimen and with symptoms treatment, or to a control group (19 patients) receiving only symptoms treatment. Clinical follow-up included the evaluation of extract tolerance, the presence of symptoms and the need for treatment to control them, and possible modifications in the following in vitro parameters: specific IgE and IgG4, and interleukins (IL-2-4-5-10-13 and IFN-γ). In the IT group, the determinations were made before the start of treatment (T0), on reaching the maximum IT dose (T1), and again after one year (T2). In the control group, the determinations were made at T0 and T2. The principal clinical results comprised a significant reduction in symptoms score at T2 (fig. 2) in the IT group that was not observed among the controls control. Likewise, a significant reduction in concomitant medication was recorded, again in favor of the IT group. Regarding the in vitro parameters, figure 3 shows a significant increase in IgG4 titers in the IT group versus the controls (p < 0.0001). There were no significant intergroup differences in terms of interleukins, however. As to tolerance, there were no local reactions, though two grade 2 systemic reactions were documented in the same patient (0.82 % of dose). With the exception of this patient, all subjects reached the maximum dosage.
Figure 2.--Symptoms evolution.
Figure 3.--Modification of the in vitro parameters.
Few studies in pediatric populations have addressed sublingual IT with an Alternaria extract29,30, though good tolerance and few reactions have been reported, as well as improvement in both the in vitro and in vivo parameters. No efficacy studies involving a double-blind, placebo-controlled design have been made, however.
An article has recently been published by the WOA (World Allergy Organization)31, specifying the guidelines for study design, patient selection, clinical planning (minimum criteria) and statistical analysis special emphasis being placed on studies in children, since these patients pose added problems such as symptoms reminder and the use of rescue medication. The article recommends the use of quality of life questionnaires, and points to the need to conduct studies in children under 5 years of age.
CONCLUSIONS
Immunotherapy with an adequately standardized extract of Alternaria alternata is well tolerated in children, even when initiation involves fast or cluster regimens. We have confirmed its efficacy in children, with reductions in symptoms and medications use. Likewise, we have recorded modifications in the in vitro parameters (IgG4). It would be necessary to confirm these results by means of efficacy studies involving an adequate design (double-blind versus placebo), in the pediatric population, and involving both the subcutaneous and sublingual routes.
ACKNOWLEDGMENT
To the partners of the work group of Inmunoterapia de la SEICAP and to Dr. Fernando de la Torre Martínez from ALK-Abelló, for their inestimable contribution to this paper.
Correspondence:
Dra. A. Martínez-Cañavate
Hospital Virgen de las Nieves
Servicio Pediatría-Alergia
Av. Fuerzas Armadas s/n
18014 Granada (Spain)
Tel.: 34-958 020 065
e-mail: anamcb@fundacionhvn.org