Chlorhexidine is widely used as an antiseptic agent. It is a potentially allergenic substance that can cause severe hypersensitivity reactions.
ObjectiveWe describe six patients who had anaphylactic reactions attributed to chlorhexidine during surgery. These patients were exposed to chlorhexidine in gels, swabs and catheters.
Materials and methodsSix patients from three UK centres with clinical history suggestive of anaphylaxis during surgery are reported. Detailed history, review of case notes, determination of chlorhexidine specific IgE, mast cell tryptase and skin tests were performed.
ResultsOn detailed assessment five of six patients demonstrated a previous history of reactions on re-exposure to chlorhexidine. All six patients had elevated specific IgE to chlorhexidine. Skin prick test with chlorhexidine was performed in four of the six patients and was found to be positive.
ConclusionImmediate hypersensitivity to chlorhexidine appears to be common but underreported in the UK. We recommend that centres investigating patients with reactions during anaesthesia and surgery should routinely include testing for chlorhexidine allergy.
Chlorhexidine is widely used in many different preparations because of its antimicrobial properties. However, it is a potentially allergenic substance, adverse reactions to which have been described in the literature for the past 30 years. Most of these reactions have been limited to the skin and were mild in severity.1–4 Type I hypersensitivity reactions, including anaphylaxis, have been reported since 1984,5 but are considered to be rare. Indeed during a ten-year period only 50 case reports of chlorhexidine-related anaphylaxis have been published.6 However, a high rate of reactions to chlorhexidine was reported in Japan and as a result specific recommendations regarding the maximum chlorhexidine concentration to be used were issued.7
There are a number of case reports describing IgE-mediated anaphylactic reactions to chlorhexidine, mostly related to anaesthesia and surgery.8–11 However the true incidence of anaphylaxis to chlorhexidine is not known and it is likely to be underestimated. In this manuscript, we describe six patients from three UK centres who have had anaphylactic reactions when exposed to chlorhexidine during surgery.
Materials and methodsSix patients from three UK centres with clinical history suggestive of anaphylaxis during surgery were investigated 6 weeks after the reaction. Informed consents were obtained from all patients. The assessment of these patients was perrofmed according to a standardised protocol in each centre and included detailed history, review of case notes, determination of total and specific IgE to chlorhexidine and other drugs used during anaesthesia, and mast cell tryptase. In addition, skin tests with chlorhexidine, latex and drugs listed in the anaesthetic record (in some case an alternative agent from the same group was used e.g. NMBA); with the exception of inhalational agents were also carried out.
The investigations were conducted in accordance with the principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines as issued by the International Conference on Harmonisation (ICH E6, 1996).
Case 1A 50-year-old man with a history of ischaemic heart disease presented for coronary artery bypass graft surgery under general anaesthesia. There was no other significant past medical or surgical history. He had no history of atopy. General anaesthesia was induced with fentanyl, etomidate, pancuronium and maintained with an air/oxygen/isoflurane mixture. Cefuroxime was given at the beginning of the procedure and the airway was secured with a Portex endotracheal tube. A central venous catheter was inserted and an infusion with saline and then Volprex was commenced. Ten minutes after insertion of the central line the patient developed flushing, an urticarial rash on his legs, facial swelling and had hypotension (70/40mmHg) and tachycardia (146beats/min). His oxygen saturation was 90% while on 100% oxygen ventilation. A diagnosis of anaphylaxis was suspected and he was promptly resuscitated. Volprex was thought to be the culprit and was discontinued. He did not respond to i.v. metaraminol or phenylephrine and was treated with bolus dose and then infusion of noradrenalin (NA). After 10min his blood pressure and pulse returned to normal and the operation continued.
He had a further reaction in the recovery room with hypotension, tachycardia and generalised urticaria. Central line catheter was withdrawn at this stage. He was treated with i.v. chlophenamine (10mg) and hydrocortisone (100mg) and maintained on small dose of i.v. NA for 12h and he subsequently made a good recovery. Blood samples taken for mast cell tryptase at 1, 2, and 12h after the event showed raised levels at 1 and 2h (17.3 and 20.2 respectively; normal range 0–14mcg/L). At 12h the MCT level returned to normal (7.1mcg/L).
The patient was referred to the allergy clinic where he underwent detailed assessment. Specific IgE for chlorhexidine was markedly raised at 30kUA/L (normal <0.35). Specific IgE for latex, suxamethonium, and quaternary ammonium compound were negative. Skin prick and intradermal tests with cefuroxime and suxamethonium were negative.
It is most likely that the anaphylactic reaction experienced by this patient was caused by chlorhexidine in the impregnated central line catheter.
Case 2A 78-year-old man with a history of ischaemic heart disease presented for an angiogram with contrast. Anaesthesia was induced with midazolam, fentanyl, and propofol and maintained with an air/oxygen/isoflurane mixture. Ten minutes into the procedure he developed breathlessness and generalised urticaria. He was treated with i.v. chlophenamine and hydrocortisone and subsequently made a good recovery. He was not referred for further investigations and was labelled as having a “reaction to the angiogram dye”. No investigations were performed during the reaction.
Few months later, he underwent an angioplasty and because of his previous reaction he was pre-medicated with prednisolone. The anaesthesia was induced with midazolam, fentanyl and propofol and maintained with an air/oxygen/isoflurane mixture. However, 10min later he developed generalised urticaria and breathlessness that responded well to i.v. chlorphenamine and hydrocortisone and the procedure was abandoned. Blood samples taken 1h after the event showed normal level of mast cell tryptase (4.6mcg/L).
He was referred to the allergy clinic for further assessment. On review it was revealed that he had had generalised urticaria in the past on exposure to Corsodyl mouthwash, which contains chlorhexidine. Specific IgE to chlorhexidine was positive at 2.3kUA/L while IgE to latex, iodine, pholcodine and skin test to lidocaine were all negative.
It was concluded with a high degree of certainty that the cause of his reaction on both occasions was allergy to chlorhexidine. We recommended chlorhexidine avoidance and the patient subsequently had a successful angioplasty.
Case 3A 72-year-old male presented for cystoscopy under general anaesthesia. Pre-operative assessment had revealed no significant medical problems and no history suggestive of atopy. General anaesthesia was induced with fentanyl, propofol, ondansetron and the maintained with isoflurane in oxygen and nitrous oxide. Gentamicin was given during the procedure and the intraoperative course was entirely unremarkable. At the end of the procedure, Instillagel® (chlorhexidine gluconate 0.25% and lidocaine 2%) was used to facilitate the passage of a urinary catheter and the patient was transferred to the recovery room. Ten minutes later, he developed hypotension (73/40mmHg), tachycardia (102beats/min) and generalised urticaria. He had no respiratory compromise. An allergic reaction was suspected and he was treated with i.v. chlorphenamine (10mg) and hydrocortisone (100mg) and 500mL of Volulyte. No blood samples were obtained for mast cell tryptase soon after the event by the anaesthetic team.
The patient made a good recovery and was referred to the allergy clinic for further assessment. On his initial assessment at the allergy clinic it was noted that he had a history of collapse and generalised pruritus following cystoscopy 30 years prior to his most recent reaction which was treated with chlorphenamine. The cause of the reaction was not investigated.
Results of the tests performed when he attended the allergy clinic demonstrated high specific IgE to chlorhexidine (4.4kUA/L). Furthermore, skin testing showed wheal reaction of 4mm to histamine (positive control), 9.5mm to chlorhexidine 0.5% and 6.5mm to Instillagel® indicative of a positive test. Results of other tests including specific IgE to latex, mast cell tryptase and skin tests with lidocaine and gentamicin were all negative.
Based on his clinical history and the results of the tests, it was concluded that his reaction was caused by chlorhexidine component of Instillagel®.
Case 4A 73-year-old male presented for a resection of bladder tumour under general anaesthesia. He had essential hypertension which was well controlled on medications and had had an uneventful insertion of a pacemaker 3 years previously. There was no personal or family history of atopy. General anaesthesia was induced with fentanyl, propofol and maintained with isoflurane in oxygen and nitrous oxide. Gentamicin and ondansetron were given during the procedure. Within 20min his oxygen saturation had fallen to 93% and he was given more oxygen. At the end of the procedure he was sweaty and confused. He was moved to the recovery room where his blood pressure was found to be 65/35mmHg, pulse 132beats/min and he vomited. He had no chest pain or dyspnoea. His pacemaker was checked and it was found to be working satisfactorily. His ECG, chest X-ray and troponin T did not reveal any abnormalities. He was treated with 200mg of i.v. phenylephrine and 500mL of Volulyte and his blood pressure returned to normal values over 10min. Blood samples taken for mast cell tryptase showed raised levels at 1 and 2h after the event (32.6 and 40.2mcg/L respectively) and was within normal limits at 18h (10.4mcg/L). He made a good recovery and was referred to the allergy clinic for further investigation.
Detailed assessment at the allergy clinic revealed that he had had a TURP operation in May 2010 and he was put on a regime of bladder instillations. Within 30min after the second procedure he developed a swollen face and generalised rash that was treated successfully with antihistamine. Various investigations were performed including blood and skin tests. Results of these tests showed raised chlorhexidine specific IgE (3.32kUA/L) and a positive skin prick test to histamine (5mm), chlorhexidine at a concentration of 0.5% (10.5mm) and 0.05% (7.2mm). His specific IgE to latex and skin prick test to latex and gentamicin were all negative.
Based on his history and test results it was concluded that his reaction had been caused by chlorhexidine exposure during surgery.
Case 5A 73-year-old man underwent an operation for resection of bladder tumour and prostate biopsy under general anaesthesia. Induction of anaesthesia was performed with fentanyl and propofol. At the end of the procedure a urinary catheter was inserted using Instillagel (chlorhexidine gluconate 0.25% and lidocaine 2%) and the patient was transferred to the recovery room, where 10min later he felt hot and itchy and had generalised urticaria and difficulty in breathing. His BP was found to be 54/34mmHg, and his oxygen saturation was 92% while on 100% oxygen ventilation.
A diagnosis of anaphylaxis was suspected and he was promptly resuscitated and treated with i.v. adrenaline, chlophenamine (10mg) and hydrocortisone (100mg). Mast cell tryptase obtained at 4h post-event was raised at 35.8mcg/L (normal range 0–14) that normalised (6.95mcg/L) by next day in intensive care. He made a good recovery and was referred to the allergy clinic for further investigation.
Detailed assessment at the allergy clinic revealed that a month previously, he had had a urinary catheter inserted with Instillagel and remembered feeling unwell and light-headed while driving back home.
A skin prick test was performed and was found to be positive to: histamine at 5mm; chlorhexidine gluconate in concentrations 0.005% and 0.05% at 4mm both; chlorhexidine digluconate 0.2% at 12mm at 30min.
Specific IgE to chlorhexidine was at 11.8kUA/L. Results of other tests including specific IgE to latex and skin tests with lidocaine were all negative.
It is most likely that the anaphylactic reaction that this patient experienced was caused by chlorhexidine, component of Instillagel, used for insertion of the urinary catheter.
Case 6A 60-year-old male presented for a cystoscopy under general anaesthesia. Pre-operative assessment revealed no significant medical problems and there was no history of atopy. General anaesthesia was induced with fentanyl, propofol, ondansetron and then maintained with isoflurane in oxygen and nitrous oxide. At the end of the procedure, Cathejell with lidocaine (chlorhexidine 0.05% and lidocaine 2%) was used.
In the recovery room, 10min later, he developed generalised urticaria, but no cardio-pulmonary compromise.
An allergic reaction was suspected and he was treated with i.v. chlorphenamine and hydrocortisone. No blood samples were obtained for mast cell tryptase during or after the event. He made a good recovery and was refereed to the allergy clinic for further assessment.
On his initial review at the allergy clinic it was noted that he had had contact urticaria to Savlon antiseptic wipe (chlorhexidine and cetrimonium) to clean a cut on his leg 4 years earlier. 15 years ago, he had developed generalised urticaria and angio-oedema 48h after receiving iodine based contrast medium for an IVP procedure.
Skin tests to lidocaine were negative, while skin prick test with chlorhexidine 0.2% produced a 4mm wheal and flare response that was delayed until 30min. Specific IgE to Chlorhexidine was 0.69kUA/L.
It was concluded, based on his history and test results that his reaction had been caused by chlorhexidine, component of Cathejell used for the insertion of the urinary catheter.
DiscussionWe report six patients from three UK centres who had type I hypersensitivity reactions to chlorhexidine during surgery. The reactions occurred when these patients were exposed to chlorhexidine in gels, swabs and catheters. Detailed assessment showed that in five of six patients there was a past history of reactions to chlorhexidine. All six patients had elevated specific IgE to chlorhexidine and skin prick test was positive in four patients tested.
Chlorhexidine is a synthetic cationic bis-biguanide, introduced in 1954 and is in widespread use as a disinfectant and antiseptic. In the community, chlorhexidine digluconate is found in many commercially-available products such as mouthwashes, tooth paste, plasters, dressings, ointments, suppositories, disinfectant solution, antiseptic creams and cleaning fluids. In the hospital, chlorhexidine is used in alcoholic and aqueous solutions of 0.05–4% for skin disinfection prior to venisection, surgical and dental procedures, and spinal anaesthesia and as a gel for urinary catheterisation and vaginal and rectal examinations. Recently, the Department of Health in the UK commissioned national guidelines advocating the use of chlorhexidine 2% in isopropyl alcohol 70% as the skin disinfectant of choice for central venous catheter insertion and with lidocaine as a lubricant for urethral catheterisation.11–12 Moreover, many locally agreed guidelines and protocols recommend the use of products containing chlorhexidine. In addition to the known sources of chlorhexidine, a potential cause of reactions are the hidden forms which include bactericidal coating for central venous catheters and Instillagel® – a commonly-used urethral antiseptic and anaesthetic lubricant. Furthermore, chlorhexidine diacetate is used as a preservative in products such as antacid preparations, contact lens fluid and cosmetics.
The incidence of chlorhexidine allergy is largely unknown perhaps due to the fact that chlorhexidine is not a drug, but a disinfectant agent, and is thus rarely suspected in connection with anaphylaxis. Reactions to chlorhexidine mostly manifest with pruritus, contact urticaria, contact dermatitis, photosensitive dermatitis or occasionally bronchospasm and occupational asthma.1,3,4,13 The first case of severe reaction to chlorhexidine was published in 19858 and more recent publications described anaphylaxis to chlorhexidine in connection with urological procedures.11,13–15
Our observation highlights several important points. The increasing use of chlorhexidine containing products may result in increasing the incidence of sensitisation and being a potentially allergenic substance, severe life-threatening reactions to it may become more frequent. Chlorhexidine is widely used as an antiseptic preparation and is not thought of as a common cause of anaphylaxis. Therefore, it is possible to overlook it as a causative agent of cardiovascular collapse. In case of an unexplained hypersensitivity reaction it is necessary to check whether chlorhexidine was used or was impregnated in a medical device that was used, as a coated catheter has been recognised as a possible cause.
It is very important to discontinue use of the medical device (catheter) as expeditiously as possible.
Chlorhexidine is commonly used and the majority of people are exposed to it unknowingly leading to sensitisation. It was demonstrated previously3,13,16,17 that most chlorhexidine-allergic patients have had previous mild reactions such as rash, swelling, itching, or generalised exanthema on exposure to chlorhexidine. In this report, five of six patients on detailed questioning had had previous reactions to chlorhexidine. It is important therefore to appropriately assess patients with mild reactions on chlorhexidine exposure to prevent future severe reactions. Clinical history together with specific IgE and skin testing can help to achieve the highest possible sensitivity and specificity and subsequently lead to correct diagnosis and management of chlorhexidine allergy.
Collecting appropriately-timed samples for mast cell tryptase remains an essential part of the investigation of a suspected anaphylactic reaction. Elevated serum tryptase levels are thought to be highly indicative of mast cell degranulation, as seen in the majority of systemic anaphylaxis cases.14,18–25 However, normal levels do not entirely exclude anaphylaxis as it is well documented that severe reactions can occur without detectable increase in tryptase levels. It is thought that in these cases basophile degranulation rather than the more typical mast cell activation is responsible for inducing the symptoms.11,20,21 Basophiles contain up to 700-fold less tryptase than mast cells, which may explain why there is no significant increase in MCT detected in plasma.11,20
In our first, fourth and fifth patients, mast cell tryptase were significantly elevated within 4h after the event and subsequently, normal levels were achieved 18h later. The second patient showed normal MCT level at 1h. The teams looking after the third and the sixth patients omitted taking blood samples for mast cell tryptase at the time of the reaction. All patients had normal base line tryptase level when assessed subsequently in three allergy clinics (Table 1).
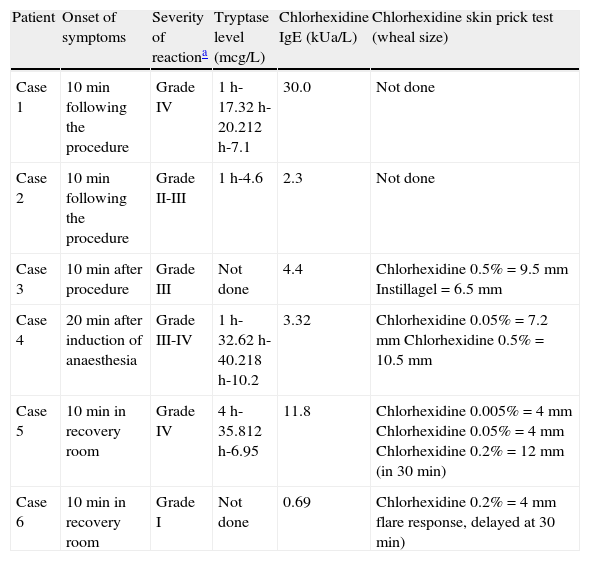
A summary of patients’ reactions and investigations.
| Patient | Onset of symptoms | Severity of reactiona | Tryptase level (mcg/L) | Chlorhexidine IgE (kUa/L) | Chlorhexidine skin prick test (wheal size) |
| Case 1 | 10min following the procedure | Grade IV | 1h-17.32h-20.212h-7.1 | 30.0 | Not done |
| Case 2 | 10min following the procedure | Grade II-III | 1h-4.6 | 2.3 | Not done |
| Case 3 | 10min after procedure | Grade III | Not done | 4.4 | Chlorhexidine 0.5%=9.5mm Instillagel=6.5mm |
| Case 4 | 20min after induction of anaesthesia | Grade III-IV | 1h-32.62h-40.218h-10.2 | 3.32 | Chlorhexidine 0.05%=7.2mm Chlorhexidine 0.5%=10.5mm |
| Case 5 | 10min in recovery room | Grade IV | 4h-35.812h-6.95 | 11.8 | Chlorhexidine 0.005%=4mm Chlorhexidine 0.05%=4mm Chlorhexidine 0.2%=12mm (in 30min) |
| Case 6 | 10min in recovery room | Grade I | Not done | 0.69 | Chlorhexidine 0.2%=4mm flare response, delayed at 30min) |
Severity grades of anaphylaxis according to Ring and Messmer criteria:
Grade I: Dermal (pruritus, flushing, urticaria, angio-oedema).
Grade II: Dermal (pruritus, flushing, urticaria, angio-oedema), abdominal (nausea, cramping), respiratory (rhinorrhoea, hoarseness, dyspnoea), cardiovascular (tachycardia (>20bpm); blood pressure change (>20mmHg systolic); arrhythmia).
Grade III: Dermal (pruritus, flushing, urticaria, angio-oedema), abdominal (vomiting, defecation, diarrhoea), respiratory (laryngeal oedema, bronchospasm, cyanosis), cardiovascular (shock).
Grade IV: Dermal (pruritus, flushing, urticaria, angio-oedema), abdominal (vomiting, defecation, diarrhoea), respiratory (respiratory arrest), cardiovascular (cardiac arrest).
A rise in tryptase levels can be quantified in serum or plasma within 30min after onset of the allergic symptoms. As tryptase's half-life is about 120min, and levels gradually decrease over time, sampling is recommended 60–120min after onset of symptoms.14,20,22 However, the level may still be raised for several hours after the reaction and therefore blood samples taken up to 6h afterwards may still be of value.11,20–24
Similarly to a previous Danish study14,20 the specific IgE levels at the time of the reaction show a great individual variation. Moreover, it was demonstrated that the levels decline over time with varying rate and not related to initial IgE level or the severity of the reaction.14,20 Analysis of IgE to chlorhexidine could be performed on the same sample taken for tryptase levels at 1–4h after the event. However, the IgE levels at the time of reaction may not reflect the true level because of the possibility of temporarily depletion of the specific IgE.14,21 Therefore IgE sampling should be postponed until 6 weeks after the acute event and preferably within six months of the reaction.14
Other tests that can be used in the diagnostic process include skin prick and intradermal skin tests with chlorhexidine gluconate at concentration of 0.5% and 0.0002% respectively.14 Furthermore, histamine release test (HR test) for chlorhexidine has been shown to correlate with the levels of the specific IgE and the skin prick test positivity.14,20 Therefore, HR test could be good adjunct to the diagnosis of chlorhexidine allergy, especially in patients with a highly suggestive clinical history, normal specific IgE and negative skin test for chlorhexidine. However, if the history is highly suggestive of anaphylaxis in the presence of raised IgE to chlorhexidine, further testing including skin testing may not be required.
The true incidence of chlorhexidine allergy is unknown. However, we believe that it is more prevalent and cases may have been overlooked due to the nature of the reaction and lack of suspicion of chlorhexidine as a culprit. Clinical history, measurement of mast cell tryptase and chlorhexidine specific IgE, with or without skin testing, help in the diagnostic process of this increasingly recognised allergy. Health care professionals should be made aware of chlorhexidine as a potential allergen and in cases of suspected anaphylaxis where the offending agent is not clearly obvious, chlorhexidine-impregnated medical devices should be immediately withdrawn. In addition, centres investigating patients with allergic reactions during a dental or a surgical procedure should routinely include testing for chlorhexidine.
Patients who report reactions, however minor, in connection with exposure to chlorhexidine should be referred to a proper allergy service for further assessment. Patients with confirmed allergy to chlorhexidine should strictly avoid all chlorhexidine containing products. Alcohol-only antiseptic and alcoholic povidone–iodine are effective in reducing healthcare-associated infections and bacterial flora of the skin26 and can be used as alternative disinfectants in patient with chlorhexidine allergy.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors declare no financial or commercial conflict of interest.
We wish to thank the immunology nursing and laboratory staff for their help and assistance.