Murine models have been widely used in the study of allergy as sensitized mice can produce IgE and/or IgG1in response after the injection of an antigen/adjuvant combination. Ailanthus altissima pollen (AAP) has been recently reported as an emerging aeroallergen in Iran. So far, several AAP candidate allergens by the screening of allergen-specific IgE in the sera from AAP sensitized patients in Iran.
ObjectiveThe aim of the present study was to detect and compare the allergens eliciting an IgE response in a mouse model, and in human, using pollen extract of A. altissima and an immunoproteomics based approach.
MethodsThe pollen proteins were extracted in phosphate-buffered saline (PBS). Thirty male BALB/c mice were randomly divided into two groups of AP extract sensitized and sham that respectively received AAP PBS extract and a PBS control by intraperitoneal injections at regular intervals. The optimized AAP protein extracts were analyzed using 2D-gel electrophoresis and were subsequently confronted to pooled sera of sensitized mice.
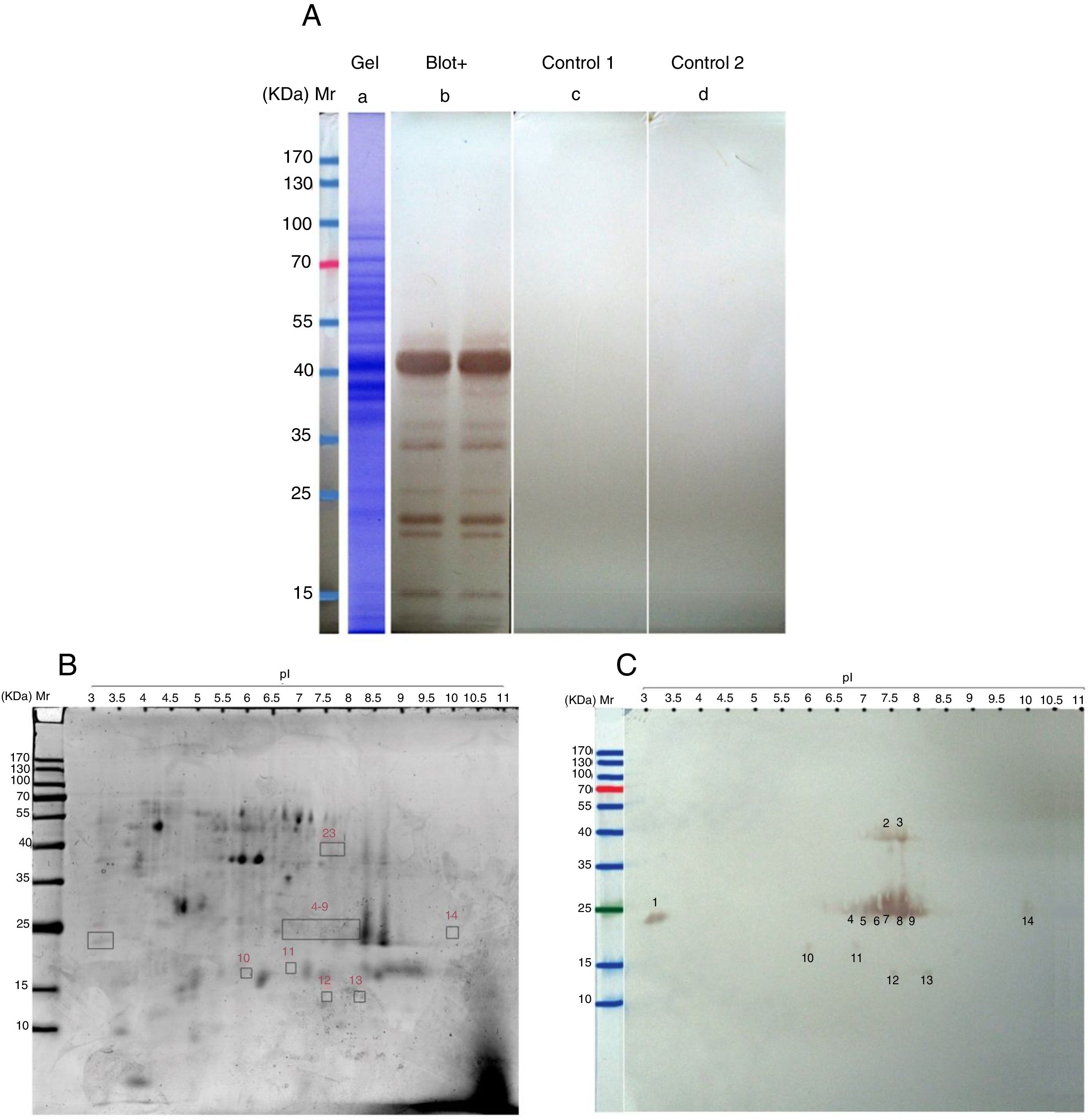
ResultsTwo-D gel electrophoresis of AAP extract allowed the separation of 125 protein spots distributed in a wide range of pI and molecular masses. Two-DE immunoblotting using pooled sera of sensitized mice led to the detection of 14 IgE reactive spots with molecular masses ranging from 12 to 40–42kDa.
ConclusionThe results do not correlate with our previous analyses using human AAP-sensitized sera. These findings reflect some differences in the sIgE reactivity to allergenic proteins in animal models.
Seasonal allergic rhinitis is a serious health problem that impacts the quality of life of people living in pollen-rich areas.1 Pollen as an important aeroallergen is the cause of about 40% of allergic diseases worldwide and pollinosis to tree pollens has increased in the arid and subtropical regions of the world.2 Penetration of inhaled pollen grains into the respiratory tract results in immediate hypersensitivity reactions (type I hypersensitivity) known as allergy.3
Many important allergic diseases are common between humans and animals, so animal models have a special place in clinical research and over 95% are conducted in mice.4,5 Murine models have been widely used in the study of allergy as sensitized mice can produce IgE and/or IgG1in response after the injection of an antigen/adjuvant combination.6 Balb/c may produce high IgE levels of these antibodies due to the type of immune response of this strain and are considered in allergenic sensitization experiments.7
Tree of heaven (Ailanthus altissima) pollen (AAP) has been reported as a common allergic pollen based on the prick test in the Middle East, including Iran.8 Recently, we reported several AAP candidate allergens by the screening of allergen-specific IgE in the sera from AAP sensitized patients in Iran.9 The aim of the present study was to detect and compare the allergens eliciting an IgE response in a mouse model, and in human, using pollen extract of A. altissima.
Material and methodsExperimental animalsThirty male BALB/c mice, six weeks of age weighing 20–21g were purchased from Pasteur Institute of Iran (IPI). Animals were housed in a standard environment (at 23±1°C with a 12h light/12h dark cycle) and fed with a maintenance diet and water ad libitum. Experiments were performed according to the national animal protection guidelines and were approved by the Ethics Committee of Pasteur Institute of Iran and conform to the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Immunization of Balb/c miceThirty male BALB/c mice were randomly divided into two groups of AAP extract immunized and control that received PBS. The AP extract immunized group were intraperitoneally injected with 130μg AAP extract adsorbed to 130μg Al(OH)3 diluted PBS in a total volume of 200μl. This dose was repeated without adjuvant on days 10 and 20. One they after the last injection, blood samples were drawn directly from the hearts of Balb/c mice and sera were stored in aliquots at −20°C until use.10
Pollen samplingA. altissima pollens were directly collected from a male tree planted in an urban green space of Tehran, Iran, during the pollination period from Mid-April to Mid-May 2014. Collected pollen grains were then sieved through an appropriate mesh and the purity checked by optical and electron microscopy was 99%. Pollen samples were then stored at −20°C until use.
Preparation of total protein extractionFifty mg (1/20wt/vol) of defatted AAP were incubated under stirring in 1mL phosphate buffer saline (PBS) pH 7.4 at 4°C overnight. The suspensions were centrifuged at 12,000g for 30min at 4°C and the supernatant was dialyzed against distilled water. The total protein content was determined using Bradford protein assay and the extraction yield of pollen extracts was calculated as previously described.11
SDS-PAGESDS-PAGE was performed according to Laemmli.12 Extracted proteins were separated under reducing conditions using 10% SDS-PAGE gels. Forty microgram proteins were loaded per lane. A reference protein standard (Fermentas, St. Leon-Rot, Germany) was applied to estimate the molecular weight of the visualized protein bands. The gel was partly electro-transferred to a polyvinylidene difluoride (PVDF) membrane for western blotting assays and another part was stained with 0.1% Coomassie Brilliant Blue R-250 (CBB-R250, Merck, Darmstadt, Germany).
Two-dimensional gel electrophoresis (2-DE)Isoelectric focusing (IEF) was performed using immobilized gradient (IPG) strips, 7cm pH 3–11 NL (GE Healthcare, Uppsala, Sweden). Strips were rehydrated passively overnight (14h) in 130μg of AAP protein extracts mixed with 125μl of rehydration buffer containing 6M urea, 2M thiourea, 4% CHAPS (w/v), 2% DTT (w/v), 1.6% IPG buffer or carrier ampholytes and 0.4% bromophenol blue stock solution. IEF was performed in an EttanIPGphor 3 system (GE Healthcare) at 20°C and a maximum current of 50μA/strip. After IEF, the strips were equilibrated for 20min with equilibration buffer 1 (6M urea, 0.003% Tris–HCl buffer pH 8.8, 29.1% glycerol, 2% SDS and 1% dithiothreitol) and then strips were equilibrated for 20min with equilibration buffer 2 (6M urea, 0.003% Tris–HCl buffer pH 8.8, 29.1% glycerol, 2% SDS and 1.25% iodoacetamide). Electrophoretic separation in the second dimension was performed by laying strips on 12% SDS-PAGE gels (running at a constant voltage of 60V for 3h). 2D gels were run in duplicate and electrotransferred onto a PVDF membrane or stained with CBB-G-250. The molecular mass of protein bands was estimated using protein markers of known molecular weight (Fermentas).
Western blotFollowing the electroblotting of SDS-PAGE and 2-DE separations, PVDF membranes were dried and blocked with 2.5% defatted milk in TBST (Tris-buffered saline and 0.1% Tween 20, PH=7.4) for 1h at room temperature (RT). Membranes were then incubated overnight with pooled sera from AAP-immunized mice (diluted 1:10 in 2.5% defatted milk in TBST) at 4°C under shaking. After several washings, membranes were incubated during 1h with 1:10,000 dilution goat poly anti-mouse IgE horseradish peroxidase (HRP) secondary antibody (Koma Biotech, Seol, Korea) followed by 3,3-diaminobenzidine (DAB) substrate kit (Sigma-Aldrich, St Louis, Mo, USA) for visualization of IgE binding bands and/or spots.
ResultsThe total protein concentration and extraction yields of AP extract were 4.3μg per μl and 77.4mg protein/g pollen respectively. The AAP extract SDS-PAGE pattern revealed 17 protein bands ranging from 10kDa to 100kDa. 1DE-Immunoblotting using pooled sera of AP extracts immunized mice revealed a major IgE-binding component of approximately 42kDa and several minor IgE-binding proteins ranging from 12 to 37kDa (Fig. 1A). Two-D gel electrophoresis of AAP extract allowed the separation of 125 protein spots distributed in a wide range of pI and molecular masses (Fig. 1B). Two-DE immunoblotting using pooled sera of sensitized mice led to the detection of 14 IgE reactive spots with molecular masses ranging from 12 to 40–42kDa, including one very acidic (spot 14), three acidic (spots 4, 10 and 11), one neutral (spot 5), eight basic (spots 2, 3, 6–9, 12 and 13) and one very basic (spot 14) protein spots (Fig. 1C).
(A) SDS-PAGE and immunoreactivity of Ailanthus altissima pollen (AAP) extract. a, Coomassie Brilliant Blue stained SDS-PAGE of the PBS extract of AAP. b–d, SDS-PAGE immunoblots of AAP extract. b, probed with the pooled sera from AAP-immunized mice. C, without serum (control 1). d, probed with the pooled sera from sham-immunized mice (control 2). (B and C) Two-DE and IgE immunoblot of A. altissima pollen (AAP) extract. B, Coomassie-stained 2-DE gels separation of AAP extract. C, two-DE IgE immunoblot of AAP extract probed with the pooled sera from AAP-immunized mice. M, molecular weight marker (Fermentas, St. Leon-Rot, Germany).
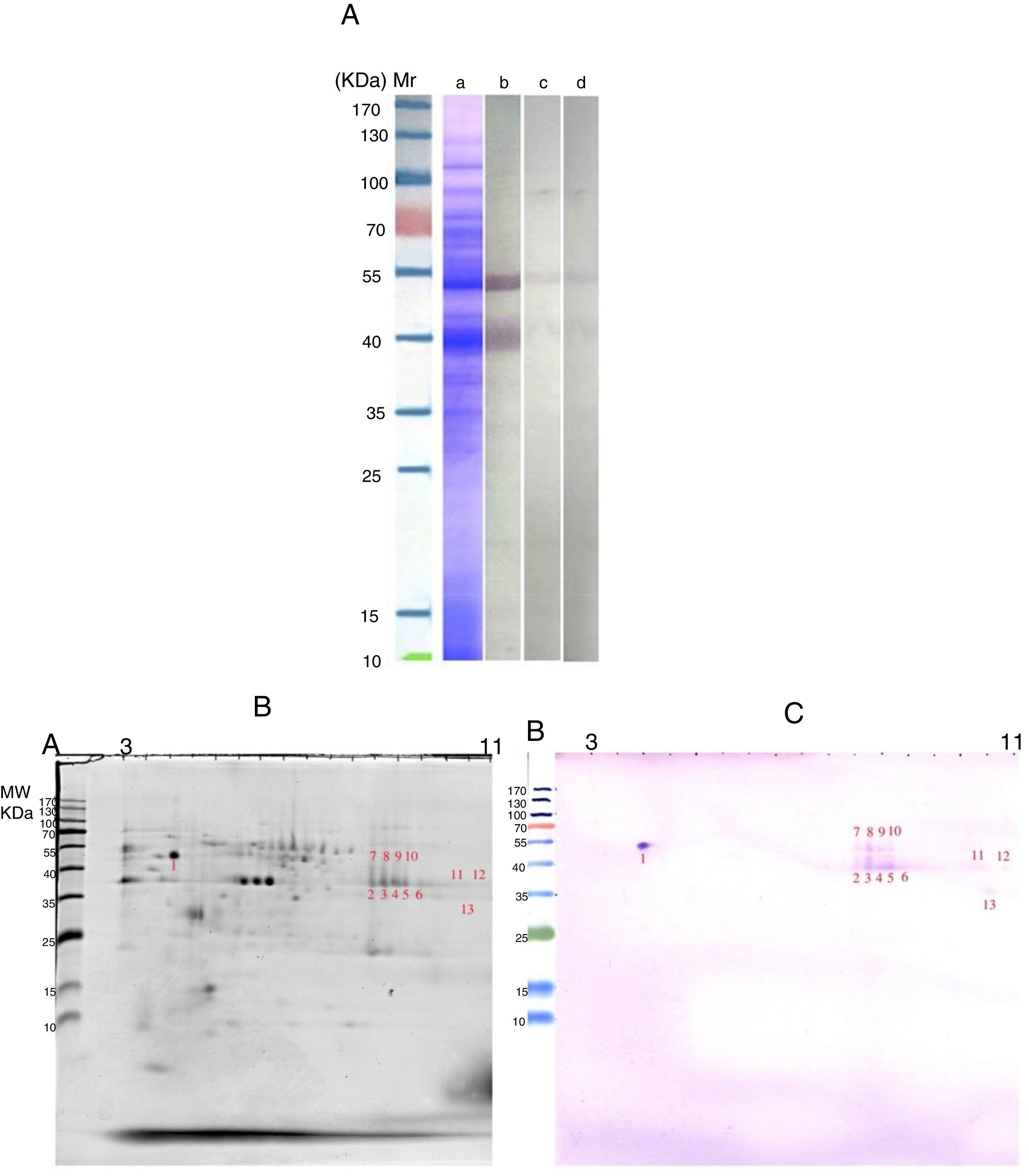
Most of our knowledge about the immune response to allergens is derived from the study of animal models and the use of murine models of allergic sensitization has increased in recent years.13 According to this, Wiedermann et al.14 reported that Balb/c mice produce high allergen-specific IgE and IgG1 levels in response to intraperitoneal injections of recombinant (r) Bet v 1 adsorbed to Al(OH)3 at regular intervals followed by an aerosol challenge with the whole birch pollen.15 Here, we developed a mice model of allergic sensitization to detect the potential allergens of AAP. The previous studies by our group9,11 provided new insights into the specific IgE response to AAP proteins in an allergic patient. Comparison IgE-reactive bands and spots using pooled sera of immunized mice (Fig. 1) with AAP extract and IgE-reactive bands and spots using an AAP positive patient's serum (Fig. 2) revealed significant differences. SDS-PAGE immunoblotting using pooled sera of AAP-immunized mice detected a major IgE-binding component of approximately 42kDa and several minor IgE-binding proteins ranging from 12 to 37kDa (Fig. 1A). However, IgE reactive bands using an AAP positive patient's serum showed a strong and clearly distinct response to two proteins of 42 and 52kDa (Fig. 2A). Although it seems that 42kDa protein band will be shared between the two human and animal serums, but doing the 2-DE immunoblotting revealed that there is no sharing between IgE reactive spots of two type serum (Figs. 1C and 2C). In the other words, the isoelectric point of IgE reactive spots within the molecular weight range 42kDa in human study was different from the ones in animal study. In general, the conventional protocol using animal sensitization with a mixture of pollen extracts and adjuvant (usually systemic e.g. intraperitoneal alum) fails to model the etiology and natural history of human respiratory allergy, which develops over time through multistep processes. Moreover, the use of alum in BALB/c mouse models of sensitization usually lead to high titters of IgG1 which do not necessarily correlate with IgE. As such, this antigen sensitization model could not be entirely representative of the repeated exposure by inhalation affecting the human respiratory mucosa. Thus, the murine immune response does not have to correlate with the human and any protein in the extract could give rise to antibodies, but the proteins recognized do not need to be the allergens in humans.16
(A) SDS-PAGE and immunoreactivity of Ailanthus altissima pollen (AAP) extract adapted from Ref. 4,8. a, Coomassie Brilliant Blue stained SDS-PAGE of the PBS extract of AAP. b–d, SDS-PAGE immunoblots of AAP extract. b, probed with the patient's serum; c, without serum, d, a non-allergic serum. (B and C) Two-DE and IgE immunoblot of A. altissima pollen (AAP) extract. B, Coomassie-stained 2-DE gels separation of AAP extract. C, two-DE IgE immunoblot of AAP extract probed with the serum of an AAP allergic patient. M, molecular weight marker (Fermentas, St. Leon-Rot, Germany).
Therefore, the present results do not correlate with our previous analyses using human AAP-sensitized sera. These pertain mainly to the higher complexity of the immune-complex and FcγRs in humans. FcγRs bind the most common class of antibody, IgG. However, no human FcγR binds human IgE, whereas 3 of the 4 mouse FcγRs namely FcγRIIB, FcγRIII, FcγRIV bind mouse IgE. These findings may partly explained differences observed in the sIgE reactivity to allergenic proteins in murine models.
Conflict of interestThe authors have no conflict of interest to declare.