Asthma and atopy, classically associated with hyper-activation of the T helper 2 (Th2) arm of adaptive immunity, are among the most common chronic illnesses worldwide. Emerging evidence relates atopy and asthma to the composition and function of gut microbiota composition. Moreover, certain gut microbial strains have been shown to inhibit or attenuate immune responses associated with chronic inflammation in experimental models. Although still a relatively nascent field of research, evidence to date suggests that the gut microbiome may represent fertile targets for prevention or management of allergic asthma and other diseases in which adaptive immune dysfunction is a prominent feature. The oral probiotics/prebiotic represents a possible therapeutic for improving asthma and allergic disease. Especially, recent technological developments that permit identification of microbes and their products using culture-independent molecular detection techniques. In this review, we literaturely summarise the aggravation or improvement of metabolic diseases by role of gut microbiota, probiotics/prebiotic treatment.
Allergy and Asthma are two major public health problems in industrialised nations, such as the United States.1–8 Both diseases are chronic inflammatory disorders caused by aberrant immune responses against common “innocuous” environmental antigens (allergens) in susceptible individuals.9 An enhanced T helper (Th)2 immune response and the elaboration of cytokines such as interleukin (IL)-4, IL-13, and IL-5 contribute to the induction and maintenance of these diseases.10 Often, atopic dermatitis is the first manifestation of atopy in infants who will develop hay fever or asthma in later childhood. The largest and earliest source of microbial exposure in human subjects comes from the intestinal tract. The gut contains a large and diverse population of microbes that is, quantitatively, the most important postnatal source of microbial stimulation of the immune system.11,12 Current evidence supports a role for gut colonisation in promoting and maintaining a balanced immune response in early life.13 Hence disruption of this process early on in life at a time of dynamic changes14,15 in the infant's gut might have long-term health effects. Both asthma16 and obesity17–19 often begin in early childhood, when the gut microbiota is primarily developed. Recent studies in animal models and in human subjects have found that an altered or less diverse gut microbiota composition has been associated with asthma and allergic disease diseases (Table 1).20–26 Moreover, certain gut microbial strains have been shown to inhibit or attenuate immune responses associated with chronic inflammation in experimental models. However, there has been no fully adequate longitudinal study of the relation between the neonatal gut microbiota and the development of asthma and allergic disease. The emergence of promising experimental studies has led to several clinical trials of probiotics (live bacteria given orally that allow for intestinal colonisation) in human subjects. Probiotic trials thus far have shown a consistent preventive or therapeutic effect on asthma and allergic disease. Because previous trials of probiotics have been limited by small sample size, short duration of follow-up, or lack of state-of-the art analyses of the gut microbiota, a lot of research needs to be done in the future. In this review we first summarise recent research about the relation between the gut microbiota and allergy/asthma, effect of probiotics/prebiotic treatment on asthma and allergic disease.
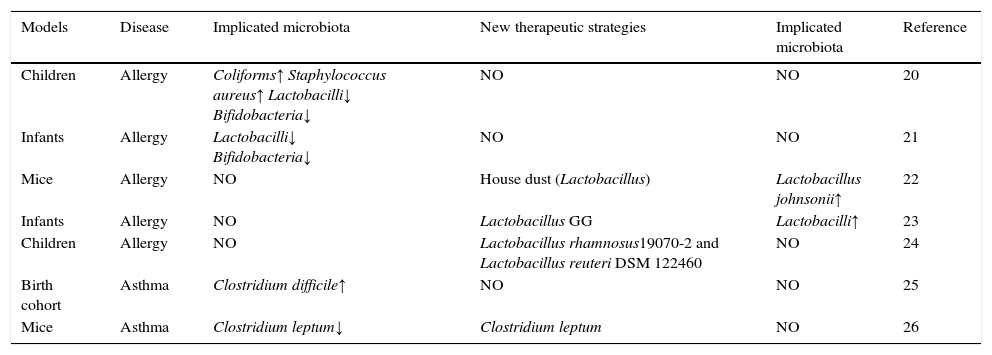
Changes in microbiota composition associated with allergy/asthma and novel therapeutic strategies.
| Models | Disease | Implicated microbiota | New therapeutic strategies | Implicated microbiota | Reference |
|---|---|---|---|---|---|
| Children | Allergy | Coliforms↑ Staphylococcus aureus↑ Lactobacilli↓ Bifidobacteria↓ | NO | NO | 20 |
| Infants | Allergy | Lactobacilli↓ Bifidobacteria↓ | NO | NO | 21 |
| Mice | Allergy | NO | House dust (Lactobacillus) | Lactobacillus johnsonii↑ | 22 |
| Infants | Allergy | NO | Lactobacillus GG | Lactobacilli↑ | 23 |
| Children | Allergy | NO | Lactobacillus rhamnosus19070-2 and Lactobacillus reuteri DSM 122460 | NO | 24 |
| Birth cohort | Asthma | Clostridium difficile↑ | NO | NO | 25 |
| Mice | Asthma | Clostridium leptum↓ | Clostridium leptum | NO | 26 |
NO: no test or no research.
Literature search was performed by using the PubMed database, the keywords used were gut microbiota (52 searches) and allergy/asthma (169 searches). Of these, 49 articles were shortlisted which discussed relation of gut microbiota and allergy/asthma. These articles were consulted for this review.
ResultGut microbiota: definitionIntestinal epithelium, mucosal immune system, and bacterial and non-bacterial flora represent a morph-functional system on dynamic balance responsible for the host local intestinal integrity and systemic barrier function.27 Gut harbour about 500 different species of microorganisms, weighing about 1.5kg in the normal subjects.28,29 The number of microbial luminal cells is 10-folds more than eukaryotic cells.28,30 At the same time, microbiota genome encode 100–1000 times more genetic information than the host genome.31 The gut, sterile during the intrauterine, is colonised immediately after birth. The numbers and species of bacteria fluctuate markedly during the early life.32 However, the gut microbiota of adults is comparatively steady over time.33 Over the course of millions of years of evolution, commensal bacteria have taken on many physiological functions essential to our health, including physical development, the intestinal barrier, immune regulation, metabolism, nutrition absorption, expelling toxin and so on.34 At the same time, gut microbiota are important factors to stimulate the mucosal immune system and systemic immune system to mature.35,36 There is convincing evidence from both human and animal studies suggesting that intestinal microbiota plays an important role in maintaining health and curing diseases; especially, new technologies have allowed the attempt to a systematic intestinal bacterial flora study giving more realistic information about its composition and its pathological variance.
Gut microbiota and allergy/asthmaAsthma is estimated to affect approximately 300million individuals worldwide, incurs significant health care expenditure,6,7 and is one of the most common chronic diseases. Given increases in disease prevalence over the last several decades, it is predicted that the number of individuals affected worldwide will increase by 100million people by 2025.8 In the United States, the risk of developing asthma is highest for children during the period between birth and four years of age37; disease prevalence is also higher among women, families below the poverty line, and people of multiple races compared to other groups.38 Although risk alleles have been associated with asthma development,39 the rapid increase in prevalence over recent decades, particularly among children, points to environmental factors playing a key role in disease development.40 Atopic sensitisation (allergy), characterised by elevated levels of total and allergen-specific IgE in the serum and typically by positive skin-prick tests to at least one of a panel of common allergens, is considered the strongest risk factor for childhood asthma development in westernised nations,41 and its rise is associated with a parallel increase in asthma prevalence.8 During the past 15 years, numerous human epidemiological studies have been conducted on the association between the gut microbiota composition and allergy/asthma. The vast majority of these studies suggest that those subjects with allergy and asthma exhibit alterations in the relative levels of “beneficial” and potentially harmful bacteria compared to healthy the subjects (Table 1).20–26 At the same time, there is convincing evidence from both human and animal studies suggesting that all kinds of factors are related to allergy and asthma disease by affecting gut microbiota. These contain early-life antimicrobial exposure, Caesarean birth, formula feeding, lack of maternal exposure to pets or livestock during pregnancy, and maternal consumption of antimicrobial during pregnancy.42–47
In 1999, Björkstén et al. reported that the research was completed in 29 Estonian and 33 Swedish 2-year-old children with either non-allergic (n=36) or had a confirmed diagnosis of allergy (n=27) to egg or cow's milk.20 In these group, the abundant levels of coliforms and Staphylococcus aureus are lower, while the levels of the lactobacilli and bifidobacteria are higher in the faecal samples compared to children with allergies to egg or cow's milk.20 Johansson et al. found that lactobacilli and bifidobacteria colonisation of infants at one week of age is a significant negative correlation with a risk for allergy at five years.21 There are some important findings about the beneficial effect of probiotics in allergy. A recent study reported that oral exposure of adult mice to house dust mediates Lactobacillus enrichment of intestinal microbiome and airway immune defence against allergens and virus infection,22 Increase of Lactobacillus johnsonii results in reductions in the total number and proportion of activated CD11c+/CD11b+ and CD11c+/CD8+ cells and airway Th2 cytokine expression.22 Meanwhile, the effects of probiotic intervention in early infancy to reduce the risk of atopic disease corroborate the above studies. Firstly, the research was completed in 230 infants with symptoms of the atopic eczema/dermatitis syndrome,23 In the whole group, the mean Severity Scoring of Atopic Dermatitis (SCORAD) decreased by 65%, especially in IgE-sensitised infants, the group with Lactobacillus GG treatment (a mixture of four probiotic strains) showed a greater reduction in SCORAD than did the placebo group,23 Treatment with LGG may alleviate the atopic eczema/dermatitis syndrome symptoms in IgE-sensitised infants but not in non-IgE-sensitised infants.23 Meanwhile, treatment resulted in increases in total counts of lactobacilli in the probiotic groups and decreases in the placebo group,23 and differences in changes between probiotic groups and the placebo group were significant. Total counts of bifidobacteria showed no major changes.23 Secondly, two probiotic Lactobacillus strains (lyophilised Lactobacillus rhamnosus19070-2 and Lactobacillus reuteri DSM 122460) were given in combination for six weeks to 1- to 13-year-old children with atopic dermatitis (AD).24 The treatment response was more pronounced in allergic patients with at least one positive skin prick test response and increased IgE levels.24 While the atopic dermatitis score and serum eosinophil cationic protein levels decreased, the production of the cytokines IL-2, IL-4, IL-10, or IFN-γ was unchanged.24 Similarly, the intestinal microbiota also play important role in development of asthma; a prospective birth cohort study show that colonisation of Clostridium difficile in gut at age one month was significantly associated with an increased risk for asthma,25 However, another study of oral administration of Clostridium leptum in adult mice showed the levels of C. leptum is negative correlation with asthma.26 Oral feeding with C. leptum for two weeks increased the percentage and total number of Tregs in the spleens and mediastinal lymph nodes and attenuated allergen-induced airway hyperresponsiveness and inflammation by inhibiting inflammatory cytokine production but enhancing interleukin 10 and transforming growth factor β1 production in the lungs.26 All in all, the data in the above shows that specific microbial species in gut may play a protective or pathogenic role in allergy or asthma development.
Concluding remarksTaken together, these findings have revealed that gut microbiota play an important role in keeping healthy and the development of allergy/asthma. The specific species of intestinal commensal bacteria may play either a protective and pathogenic role in development of allergy/asthma. Meanwhile, a number of studies completed in both animal models and humans show that an effective strategy of preventing and managing allergy/asthma might be targeting gut microbiota, Probiotics/prebiotic can confer health by modulating the composition of gut microbiota restoring the physiological bacterial flora. Many researches have provided a compelling rationale for exploring the oral administration of probiotics/prebiotic as adjunctive therapies to allergy/asthma, but the available data in this field remain limited, and the relevant scientific work has only just begun. A lot of research needs to be done in the future. Firstly, the host is big and complex environment for gut bacterial flora. It is unclear whether alteration of gut microbiota leads to the development of allergy/asthma or the happening of the allergy/asthma are the primary cause of changes of gut microbiota. An in-depth understanding of the mechanism of action between gut microbiota and host is needed. Meanwhile, it is worth noting that a great deal of research has been completed in different populations or mammals models and various types of food. Secondly, opening up an entirely new approach is to maintain health and cure allergy/asthma by monitoring and controlling gut microbiota which is very important. It is possible to monitor the human intestinal microbiota and manipulate it if required through the use of probiotics and/or prebiotics. Developing new probiotic is on the basis of the mechanism between the microbiota and allergy/asthma. At the same time, it is possible is to develop probiotic from gut microbiota of healthy group, for example, faecal transplant is a therapeutic strategy that has attracted more attention. We should make it clear how probiotics and/or prebiotics restore bacterial flora. Thirdly, as the availability of the relevant research in the area is limited more studies should be done to elucidate the interaction between the microbiota and allergy/asthma, a great deal of well-controlled clinical research with intestinal microbiota is needed to make sure of safety in patients. All in all, we have opened up an entirely new approach to the understanding and treatment of allergy/asthma, research has just started in this field, more research is urgently needed.
Ethical disclosuresConfidentiality dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest to declare.
We thank Professor Hong Zhang for critical review of this manuscript.